 HOME | ÍNDICE POR TÍTULO | NORMAS PUBLICACIÓN
HOME | ÍNDICE POR TÍTULO | NORMAS PUBLICACIÓN Espacios. Vol. 36 (Nº 24) Año 2015. Pág. 21
José Luiz Ferreira da TRINDADE 1; Brás Heleno OLIVEIRA 2; Maria Helene Giovanetti CANTERI 3; Tafael Lucas PEREIRA 4
Recibido: 30/08/15 • Aprobado: 12/10/2015
ABSTRACT: The study of plant growth regulators, such as gibberellins, has been sustained by the pursuit of increase productivity and quality of agricultural products. New fermentation processes have focused on the isolation and characterization of compounds used as plant growth regulators. The fungus Gibberella fujikuroi is the most commonly used for the production of the commercial gibberellin, the gibberellic acid, by submerged fermentation. The use of precursors is one of the strategies to increase the yield in the fermentation process. The biotransformation of tetracyclic diterpene acids with the skeleton ent-Kaurene by fungi leads to production of gibberellins. This study focuses on evaluating the conditions for the biotransformation of steviol glycosides to produce gibberellic acid. In the preparation of the inoculum strains of G. fujikuroi, s1 and s2, the replacement of glucose by steviol glycosides in culture medium promoted positive change of the fungus response in the biotransformation. In addition. After isolation, purification and characterization by NMR of this compound, assays were carried out to test its biological activity, using as control the gibberellic acid. With the aleurone test in barley. These founds indicate therefore the possibility of using the steviol glycosides as a precursor in the production of gibberellic acid. |
RESUMO: O estudo de reguladores de crescimento tais como giberelina, tem sido sustentado pela busca de aumento de produtividade e qualidade dos produtos agrícolas. Novos processos de fermentação têm-se centrado sobre o isolamento e caracterização de compostos utilizados como reguladores do crescimento de plantas. O fungo Gibberella fujikuroi é o mais vulgarmente utilizado para a produção comercial de giberelina, o ácido giberélico, por fermentação submersa. A utilização de precursores é uma das estratégias para aumentar o rendimento no processo de fermentação. A biotransformação de ácidos diterpenos tetracíclicos com o esqueleto caureno por fungos leva à produção de giberelinas. Este estudo centra-se em avaliar as condições para a biotransformação de glicosídeos de esteviol para produzir ácido giberélico. Na preparação das estirpes de inóculo de G. fujikuroi, S1 e S2, a substituição da glucose por glicosídeos esteviol em meio de cultura resultou em mudança positiva da resposta do fungo na biotransformação. Após o isolamento, purificação e caracterização por espectro de RMN deste composto, foram realizadas ensaios para testar a sua actividade biológica, utilizando-se como controlo o ácido giberélico. Com o teste de aleurona de cevada no. Estes encontrados indicam, portanto, a possibilidade de utilizar os glicosídeos de esteviol como um precursor para a produção de ácido giberélico. |
The growth of plants is based on the production of cells in meristems, followed by elongation of these new cells formed. The plant growth regulators have the role of influence in most aspects of growth, cell development and differentiation of tissues and organs of plants (Kende and Zeevaart, 1997). A large number of compounds, including hormones, have their presence in organs of the plant determined by a combination of rates of enzymatic biosynthesis and catabolism, abundant in the development processes of plants. The plant hormones are present in very small quantities in plants (Prado Neto et al., 2007)
The gibberellins (GAs) belong to an important group of growth hormones in plants, involved in the control of different physiological processes such as: adjustment of sprouting of many commercial crops, regulation of flowering in some biennial, photo induced or fruit plants, and in the ripening and fruit development (Escamilla-Silva et al., 1999; Albuquerque and Dantas, 2004). These substances belong to a group of tetracyclic diterpene acids (skeleton ent-Kaurene), present in low concentrations in plants, in which these substances regulate different stages of growth and development, constituting a family of natural compounds (Richman et al., 1999). The activity of gibberellins as natural hormones from plants occurs through of development processes control, such as induction of the enzymatic hydrolysis activity during germination, the elongation of the stem, the induction of flowering, the seed production and the development of the pericarp (Graeb, 1987).
In addition to the gibberellins, other natural constituents of plants have shown activity in the form of growth stimulation. The ent-kaurenoic acid has been reported as a natural compound with activity in tests with rice, in the same way as the steviol, highly provable due to that these compounds shown activity as a result of the conversion to a type of gibberellin in the plants (Helliwell et al., 2001). The first step in the biosynthesis of GAs corresponds to the two steps of the geranyl pyrophosphate cyclizing to ent-Kaurene, with ent-copalyl diphosphate (pyrophosphate) as a possible factor intermediary limiting of synthesis speed, both higher plants and fungi.
The highly hydrophobic ent-Kaurene is then oxidized by one or more cytochrome P450 monooxygenases for ent-kaurenoic acid by pathway ent-kaurenol and ent-kaurenal (Helliwell et al., 2001; Tudzinki, 1999). After the sources of nitrogen exhaustion, the exponential growth of the fungus ceases and the secondary metabolism is disarmed, with concomitant biosynthesis of gibberellins, mainly GA3 (Fernandes-Martins et al., 2000).
The GAs produced from fungi are part of an extensive family of other compounds found in higher plants. The studies of these fermentations have shown a large number of compounds that are clearly implicated in the gibberellins biosynthesis and breakdown (Jefferys, 1970)
Other GAs were identified and commercially produced, after initial discover, due to the Gibberella fujikuroi activity producing the GA3 (Hedden et al., 2001). Then, several studies have been conducted to reveal the conditions of fermentation for gibberellins production (Stodola et al., 1995; Surulirajan and Sarbhoy, 2000), involving metabolism study (MacMillan, 1997), its extraction (Hollmann et al., 1995) and chromatographic analysis methods (Pearce et al., 1994). Thus, the commercial production of gibberellins with the fungus Gibberella fujikuroi was probably an extensively studied industrial process at the end of the last century. During its production, there are many other secondary metabolites, initiated when all the nitrogen of the culture medium is consumed. The metabolites more important are the bikaverin and norbikaverin (Graeb, 1987; MacMillan, 1997).
The aglycone steviol of steviol glycosides is a derivative of ent-kaurenoic acid. In addition, the metabolism of GA12 varies between species or organs of the same specie with respect to the position and sequence of oxidative steps. The greatest variations between the various GAs concern to the presence or absence of nonsaturated binding in the A ring, the number and position of hydroxyl groups and the number of hydroxyl groups. The gibberellins GA4, GA1 and GA3 are some among many present in plants with possibilities of many interconversion between them (Castellaro et al., 1990).
The leaves of Stevia rebaudiana Bertoni, Compositae family, accumulates the so-called sweeteners of stevia, in proportion of 5-10% of its dry weight basis. The main components are the steviol glycosides and the rebaudioside-A (Fig 1).
|
R1 |
R2 |
Name |
H |
H |
Steviol |
|
H |
β-Glu |
Steviolmonoside |
|
β-Glu |
β-Glu |
Rubusoside |
|
H |
β-Glu (2-1)- β-Glu |
Steviolbioside |
|
β-Glu |
β-Glu (2-1_ β-Glu |
Stevioside |
|
β-Glu |
β-Glu (2-1) β-Gluβ-Glu (3-1) |
Rebaudioside A |
Fig. 1 Structure of steviol and their glucosides (Shibata et al. 1995)
Despite the importance of C-13 hydroxy-GAs in the physiology of gibberellins studies in plants, only a small amount of GAs can be obtained from them. The concentration of gibberellins in vegetative parts of plants is of the order of mG/kg, and in reproductive parts, of some tens of mg/kg of fresh plant. Because of the low concentrations, the gibberellins extraction on a commercial scale is not economically viable (Sato, 1994).
Due to the relevance of gibberellins as stimulating the growth of plants, there was a significant increase in studies of its production and isolation. The conversion of some precursors containing the hydroxyl at position C-13 in GAs by Gibberella fujikuroi is reported as extremely important. The steviol, an aglycone of steviol glycosides, obtained from leaves of Stevia rebaudiana, it might be a precursor and its metabolism by G. fujikuroi is interesting (Kim et al., 1996). The objectives of this work were to study conditions to increase the yield of GA3 in the biotransformation process by fungus Giberella fujikuroi, by adding steviol glycosides to the culture medium.
2.1. Material
The steviol glycosides in powder form, used as raw material for biotransformation, were supplied by Steviafarma Industrial S.A., located in Maringa, Parana, Brazil. We used two strains of Gibberella fujikuroi (strain s1, strain s2), from to the particular mycology collection of Natural Products and Biotransformation laboratory (Department of Chemistry at the Federal University of Parana). The conservation of strains and other samples was performed in the refrigerator Electrolux Frostfree DFF 44 - PREMIUM.
For the determination of the biological activity, the barley seeds were submerged in H2SO4, 50% for 2 hours, for the pericarp chemical peeling. Then, the acid was decanted and the shelled seeds were washed thoroughly with distilled water to remove the excess acid, peel and other debris. The clean seeds were dried in a desiccator under vacuum at room temperature overnight. In laminar flow chamber, the seeds were transversely cut to half for removal of embryos activators of enzyme activity, to avoid interference in the result. The cut pieces were irradiated with UV light for 15 minutes to eliminate any microbiological contamination.
2.2. Selection of strains
The growth media used for the selection of microorganisms was the ICI 10% with the following composition: 80 g of glucose, 2.4 g of ammonium nitrate; 5.0 g of potassium phosphate di-hydrogenated; 1 g of magnesium sulphate. 10H2O; 2 mL of STE (Solution Trace Elements); water q.s.p. 1000 ML. The weighing was made with balance MARS: model 2000 (Sao Paulo - SP), or OHAUS balance model Explorer (Pine Brook, NJ, USA). Then, adjustment was made for pH 5.0, being transferred aliquots for autoclaved bottles for 20 minutes at temperature of 121 oC and a pressure of 110 kPa man., in autoclave FABBE brand model 108 series in 6779 patients (São Paulo - SP). In the pH determination in the reaction medium, we used a MICRONAL pHmeter model B 374 (São Paulo - SP). In this process of selection were used 250 mL Erlenmeyer flasks containing 50 mL of liquid growth media, to which steviol glycosides was added at a concentration of 1 mg mL-1. For the fermentation was used rotary shaker with 120 rpm and at a temperature of 29 oC. The yield of the process was assessed through the production of biomass (mg mL-1) and GA3 (mG mL-1).
2.3. Analyses
The fermentation process was carried out in an incubator, with orbital thermostated shaking, TECNAL, model TE 421, with overheating limit control. The experiment was performed under aseptic conditions in a laminar flow vertical mark TROX FLV model series 293 (Curitiba - PR). With the objective to understand the mechanism of formation of the biomass and the rate of production of gibberellic acid during the biotransformation process, a new experiment was carried out. We evaluated the formation of the biomass and the production of GA3 by HPLC from inoculation time (zero), until the fermentation final, totaling 192 h.
Samples were collected from the test and control for analysis 48 and 96 h after initial time. The essay was stopped after 96 h in agreement with results obtained in preliminary studies. The fermented was filtered under vacuum, with successive washing with ethyl acetate (3 x 100 mL), added to anhydrous sodium sulphate. Subsequently, the solvent was its volume reduced in rotatory evaporator, resulting in an extract containing the biotransformation products. The process of extracts preparation for analysis was the ultrafiltration, thought filters MILLIPORE I. C. L. HA 0.45 m (Billerica, MA, USA), followed by Cole-Parmer of 0.2 micrometers.
The glucose quantification for fermentation monitoring was performed in spectrophotometer SHIMADZU UV 1601 (Kyoto, Japan), by the 3,5-dinitrossalicilato sodium (DNS) method. The silica Si-60 F254 (Merck, art. 5554) with thickness of 0.2 mm was used as adsorbent for qualitative analysis by Thin-layer chromatography (TLC). The chromatograms results were obtained by methanolic solution of sulfuric acid (50%) and heating and/or irradiation in UV-VIS, at 254 and 365 nm. The steviol isolation was performed from the addition of 10 g steviol glycosides, dissolved in 500 mL of water, add 10 g of sodium periodate.
This mixture was kept for 16 hours, without light. After this period, potassium hydroxide (7.5 g) was added up to pH 12 and the mixture was heated under reflux for one hour. After cooling with ice, glacial acetic acid was add until to pH 4.0, plus 40 mL of sodium bisulphite 1.0 N. The mixture was extracted with ethyl acetate (3 x 250 mL). The dried organic phase with anhydrous sodium sulfate was filtered and the solvent was evaporated in rotatory evaporator. The steviol was recrystallisated three times with methanol and compared with authentic sample of steviol by TLC eluted in mobile phase hexane:acetonitrile (1:1) (Ogawa et al.,1980).
The quantitative analysis of gibberellins and steviol in filtered extract were carried out by HPLC system Varian (Palo Alto, CA, USA); quaternary pump (model 9012Q), UV detector photodiode (model 9065) and automatic injector (model AI200) C-18 column Rainin, 5 a (4.6 X 250 mm).
The water used in the analyzes was distilled in water distiller, Pilsen FABBE brand, model 106 (Sao Paulo - SP), distilled again in glass distiller water MARS brand, model MB 10.02 (Sao Paulo - SP) and then purified in water purifier brand MILLIPORE (Sao Paulo - SP), model Simplicity 185.
For HPLC, samples were prepared by extraction in solid phase method with the the vacuum system preparation Varian, model Vac Elut 20 (Palo Alto, CA, USA) and centrifuged in microcentrifuge LABNET brand, model Force 7 (Woodbridge, NJ, USA).
2.4. Preparation of the Standard Curve for Quantification of GA3
The determination of gibberellins was made by the method of external calibration. For this, we constructed a calibration curve with solutions of GA3 in final concentrations 505 µg mL‑1; 252.5 µg mL‑1; 125.25 µg mL-1; 63.12 µg mL-1 e 31.56 µg mL-1. Each solution (20 µl) was injected of in chromatographic system and the elution was performed with acetonitrile-water (30:70) in the first period (six minutes) and acetonitrile-water (90:10) in the rest of the time (13 minutes). These solutions were analyzed by HPLC with detector adjusted to 205 nm.
2.5. Statistical Analysis
Three replicates were taken for each analysis, and the results were expressed as the mean of those values ± Sx. Analysis of variance (ANOVA) was performed at the p = 0.01 significance level to study the variation among samples. The Tukey test was used to determine differences (Canteri et al., 2001).
We tested the strains of G. fujikuroi s1 and s2, as producer of GA3, with the steviol glycosides in the biotransformation medium, in concentrations of 1.0; 2.0 and 3.0 mg mL-1, with the objective of assessing the efficiency of production in these different concentrations (Table 1). The calibration curve for quantification of GA3 was linear in the range of concentration used, with R2= 0.999730.
These tests showed that there was a statistically significant increase in the production of GA3, proportional to the increment of steviol glycosides in the fermentation medium. The pH measured at the end of the biotransformation process presented mean values in triplicate, between 4.2 and 3.6, respectively for the G. fujikuroi s1 and s2, indicating different optimal conditions for the hydrolysis of steviol glycosides. It was also observed that the G. fujikuroi s2, showed a yield in the production of GA3 between 4.3 and 4.9 times higher, compared to the control group and 3.3 times higher than the strain G. fujikuroi s1, showing greater capacity of biotransformation (steviol glycosides to GA3).
Table. 1 Evaluation of strains of Gibberella fujikuroi s1 and s 2 at pH 5 with respect to the biomass and GA3 production in different concentrations of steviol glycosides (n= 3).
Concentration of Steviol glycosides (mG mL-1) |
Biomass (mG mL-1)
|
GA3 (uG mL-1) |
||
S1 |
S2 |
S1 |
S2 |
|
Control |
5,6b |
5,2b |
3,6c |
4,0d |
1,0 |
5,6b |
6,3a |
5,1b |
17,0c |
2,0 |
5,0c |
5,2b |
5,1b |
18,4b |
3,0 |
7,7a |
5,6b |
7,2a |
19,5a |
The values are expressed as mean (n=3); Means in the same column followed by the same letter are not significantly different at the 5% probability level (Tukey' s test)
Other authors (Jefferys, 1970; Gohlwar et al., 1984, Brückner and Blechsmidt, 1991) demonstrated that the range of optimum pH for the production of gibberellins is between 3.0 and 5.0 range. Changes in this optimum range pH may change the availability of nitrogen, phosphorus and carbon sources, affecting decisively the biosynthesis of GA3, as well as to changing the lability of one or more enzymes of biosynthetic route of gibberellins.
A new experiment was carried out to prepare the inoculum for future biotransformation processes. The replacement of glucose by steviol glycosides as carbon source, in the growth media of the inoculum, had as expectation improved the GA3 production. The results of this experiment are shown in Figure 2.
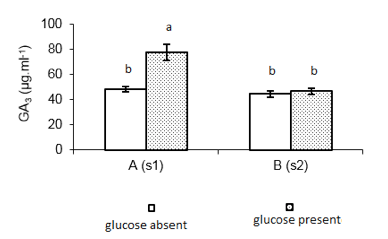
Fig 2. Production of GA3 with inoculum prepared with and without glucose (n = 3). A(s1) and B(s1) = strains G. fujikuroi s1 and G. fujikuroi s2; Control = medium ICI 10% (without steviol glycosides); Experiment = ICI 10 % + 2.0 mg. ml-1 steviol glycosides medium; Equal lowercase letters indicate statistical similarity (1% of significance), ANOVA one-way and Tukey's test
There was significant difference between the tests (Fcal= 22.40 > Fcrit= 6.98; 1% significance). Due to this fact, for continuity of the experiments was selected strain Gibberella fujikuroi S1, ICI 10% with addition of 2.0 mg mL-1 steviol glycosides at pH = 5.0, that presented the highest results.
With the objective to understand the mechanism of formation of the biomass and the rate of production of gibberellic acid along the biotransformation a new experiment was carried out. The results for biomass and GA3 production, during 192 h are shown in Figure 3.
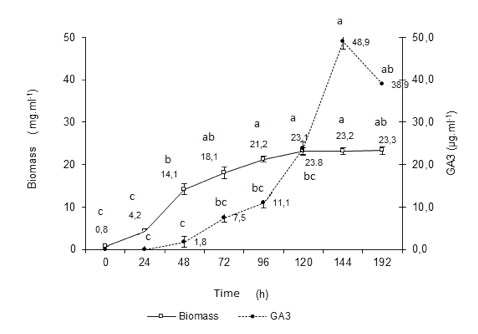
Fig. 3 Kinetics of GA3 and biomass production from the steviol glycosides (n =3). Equal lowercase letters indicate statistical similarity (1% of significance level), ANOVA one-way and Tukey's test; Fermentation with Gibberella fujikuroi s1, in ICI 10% with addition of 2.0 mg. mL-1 steviol glycosides at pH = 5.0.
The quantification of GA3 and biomass formed evidence a high and significant Pearson correlation (p< 0.05) between the values (r= 0.74). Difference statistically significant was evidenced between the times, for both: biomass production (Fcal= 34.66 > Fcrit= 4.02; 1% significance level) and concentration of GA3 (Fcal= 6.63 > Fcrit= 4.45; 1% significance). At the time of 144 h in the biotransformation process, we reached the maximum peak to both parameters. From this time, we considered that microorganisms metabolize the GA3, since the initial sources of nitrogen and carbon, placed in the growth media, were consumed along the fermentation.
If the rates of biomass increase are considered, the interval between 24 to 72 hours can be designated as the exponential growth phase of the microorganism. From that moment, up to 120 hours, as deceleration phase, followed by stationary phase up to 192 hours. We observed that 52.8% of all GA3 was formed at this stage (23.1 to 48.9 mG mL-1).
The Gibberella fujikuroi strain S1 was used in the biotransformation process of steviol glycosides in GA3 and hydroxysteviol. This strain showed higher yield, when compared to the strain S2, mainly when the inoculum was obtained in the absence of glucose in the growth media and adding steviol glycosides as only carbon source.
The ideal pH for the steviol glycosides biotransformation to GA3 is close to 4.0, also ideal pH for the hydrolysis of steviol glycosides. This indicated the biological value of this compound, which can be complemented with pilot scale tests, as a substitute for GA3, with higher ease to production and lower cost.
Albuquerque, T.C.S. and B.F. Dantas, 2004. Uso de substâncias orgânicas na produção de uvas de mesa. In: Cultivo da Videira. Brasília: Embrapa Semi-Árido. Available also at http://sistemasdeproducao.cnptia.embrapa.br/FontesHTML/Uva/CultivodaVideira/substancias.htm
Borrow, A., S. Brown, E.G. Jefferys, R.H.J. Kessell, E. C. Lloyd, P. B. Lloyd, R. V. Botelho, E. J. P. Pires and M. M. Terra, 2004. Efeitos do thidiazuron e do ácido giberélico nas características dos cachos e bagas de uvas 'niagara rosada' na região de Jundiaí-SP. Rev. Bras. Fruticultura, 25: 74 – 77.
Brückner, B. and D. Blechsmidt, 1991. Nitrogen regulation of gibberellin biosynthesis in Gibberella fujikuroi. Appl. Microbiol. Biotechnol., 35: 646-650.
Canteri, M.G., R.A. Althaus, J.S. Virgens-Filho, E.A. Giglioti and C. V. Godoy, 2001. SASM - Agri : Sistema para análise e separação de médias em experimentos agrícolas pelos métodos Scoft - Knott, Tukey e Duncan. RBAC., 1: 18-24.
Castellaro, J.S., S.C. Dolan, P. Hedden, P. Gaskin and J. Macmillan, 1990. Stereochemistry of the metabolic steps from kaurenoic acids to kaurenolides and gibberellins. Phytochem., 29: 1833-1839.
Escamilla-Silva, E.M., L. Dendooven, J.A. Uscanga-Reynell, A.I. Monroy- Ramírez, G. González-Alatorre and M.T. Martínez, 1999. Morphological development and gibberellin production by different strains of Gibberella fujikuroi in agitador orbital flasks and bioreactor. World J Microb Biot., 5: 753 – 765.
Fernandes-Martins, R.E., C. Domenech, E. Cerdá-Olmedo and J. Avalos, 2000. Ent-Kaurene and squalene synthesis in Fusarium fujikuroi cell-free extracts. Phytochem., 54: 723-728.
Gohlwar, C.S., R.P. Sethi, S.S. Marwaha and V.K. Sehgal, 1984. Gibberellic acid biosynthesis from whey and simulation of cultural parameters. Enzyme Microb Tech., 6: 312-316.
Graeb, J.E., 1987. Gibberellin biosynthesis and control. Annu Rev Plant Physiol., 38: 419-465.
Hedden, P., A.C. Phillps, M.C. Rojas, E. Carrera and B. Tudzynski , 2001. Gibberellin biosynthesis in plants and fungi: a case of convergent evolution? J Plant Growth Regul., 4: 319-331.
Helliwell, C.A., P.M. Chandler, A. Poole, E.S. Dennis and W.J. Peacock, 2001. The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc Natl Acad Sci USA., 98: 2065-2070.
Hershenhom, J., M. Zoahr, B. Crammer, Z. Ziv, V. Weinstein, Y. Kleifeld, Y. Lavan, R. Ikan, 1997. Plant-growth regulators derived from the sweetener stevioside. Plant Growth Regul., 23: 173-178.
Hollmann, D., J. Switalski, S. Geipel and U. Onken, 1995. Extractive fermentation of gibberellic acid by Gibberella fujikuroi. J Ferment Bioeng., 79: 594-600
Jefferys, E.G., 1970. The gibberellin fermentation. Adv. Appl. Microbiol., 13: 283-316.
Jones, R.L. and J.E. Varner, 1967. The bioassay of gibberellins. Planta, 72: 155-161.
Kende, H. and J. Zeevaart, 1997. The five "classical" plant hormones. Plant Cell., 9:1197-1210.
Kim, K.K., Y. Sawa and H. Shibata, 1996. Hydroxylation of ent-kaurenoic acid to steviol in Stevia rebaudiana Bertoni—purification and partial characterization of the enzyme. Arch. Biochem. Biophys., 332: 223 – 230.
MacMillan, J., 1997. Biosynthesis of the gibberellin plant hormones. Nat Prod Rep., 14: 221-243.
Ogawa, T., M. Nozaki and M. Matsui, 1980. Total synthesis of stevioside. Tetrahedron Lett., 36: 2641-2648.
Pearce, D. W., M. Koshioka, R. Pharis, 1994. Chromatography of gibberellins. J. Chromatogr. A., 658: 91-122.
Prado- Neto, M., A. C. V. L. Dantas, E. L. Vieira and V. O. Almeida, 2007. Germinação de sementes de jenipapeiro submetidas à pré-embebição em regulador e estimulante vegetal Ciênc. Agrotec., 31: 693-698.
Reeve, D. and A. Crozier. Gibberellin bioassays. In: Krishn, H. N. Gibberellins and plant growth. John Wiley & Sons, New Delhi, 1975. pp. 35-64. ISBN 978-0-47-0507971.
Richman, A.S., M. Gijzen, A.N. Starratt, Z. Yang and J.E. Brandle, 1999. Diterpene synthesis in Stevia rebaudiana: recruitment and up-regulation of key enzymes from the gibberellin biosynthetic pathway. Plant J., 19: 411-421.
Sato, C.T., 1994. Produção microbiológica de giberelinas em escala laboratorial. [Master thesis.] Maringá: Universidade Estadual de Maringá,67 pp.
Shibata, H., Y. Sawa, T. Oka, S. Sonoke, K.K. Kim, M. Yoshida, 1995. Steviol and steviol-glycoside: glucosiltransferase activities in Stevia rebaudiana Bertoni – purification and partial characterization. Arch. Biochem. Biophys., 321: 390-396.
Stiirmer, J. C., 2006. Obtenção de diterpenóides tetracíclicos com potencial ação reguladora de crescimento vegetal. [Ph. D. thesis.] Curitiba: Universidade Federal do Paraná, 110p. Available also at http://dspace.c3sl.ufpr.br/dspace/bitstream/handle/1884/7556/Tese.corrigida2.pdf?sequence=1>
Stodola, F.H., K.B. Raper, D.I. Fennell, H.F. Conway, V.E. Sohns, C.T. Langford and R. W. Jackson, 1995. The microbiological production of gibberellins A and X. Arch. Biochem. Biophys., 54: 240-245.
Surulirajan, M. and A.K. Sarbhoy, 2000. Gibberellic acid production by Fusarium moniliforme. J Mycopathol Res., 38: 101-104.
Tudzinki, B., 1999. Biosynthesis of gibberellins in Gibberella fujikuroi: biomolecular aspects. Appl. Microbiol. Biotechnol., 52: 298-310.
1. Food Department, Federal Technological University of Paraná, Ponta Grossa/PR, Brazil, e-mail: joselftri@hotmail.com
2. Federal University of Paraná, Chemical Department, Curitiba/PR, Brazil, e-mail: bho@ufpr.br
3. Food Department, Federal Technological University of Paraná, Ponta Grossa/PR, Brazil, e-mail: canteri.mhg@gmail.com
4. Food Department, Federal Technological University of Paraná, Ponta Grossa/PR, Brazil, e-mail: tafadluca@hotmail.com