 HOME | ÍNDICE POR TÍTULO | NORMAS PUBLICACIÓN
HOME | ÍNDICE POR TÍTULO | NORMAS PUBLICACIÓN Espacios. Vol. 37 (Nº 30) Año 2016. Pág. 20
Paula Emília de Souza PRATES 1; Glaucia Aparecida PRATES 2; José Claudio CARASCHI 3; Wasim Aluísio Prates SYED 4
Recibido: 31/05/16 • Aprobado: 02/07/2016
ABSTRACT: This study aims to assess the resistance to corrosion of stainless steels used in the manufacture of part of automotive exhaust systems, especially as regards ethanol fuel condensation in the rear silencer of light vehicles. One synthetic solution of ethanol fuel condensation has been formulated based on chemical analyses from specimens of natural solutions collected directly from the vehicle while running during its cold phase, that is, within the first 505 seconds. The assessment of resistance to corrosion of ferritic stainless steels AISI 439 and 409, as compared to the austenitic steel AISI 304, was carried out when in the presence of a synthetic solution of ethanol fuel condensation. The comparative analysis of the potentiodynamic polarization curves of the steels studied indicates that there is no difference in behavior concerning the corrosion of these steels. In addition, no identification of a potential of pitting within these curves could be observed. Upon implementation of Dip-Dry tests, it could be verified that the steels do in fact have a similar behavior in that they did not undergo corrosion, only oxidation. It could therefore be concluded that all the stainless steels studied can be recommended for use in the rear silencer of light vehicles and that the choice of a ferritic stainless steel would be the most economical among the three steels studied. |
ABSTRACT: Este estudio tiene como objetivo evaluar la resistencia a la corrosión de los aceros inoxidables utilizados en la fabricación de una parte de los sistemas de escape de automóviles, especialmente en cuanto a la condensación de combustible de etanol en el silenciador trasero de los vehículos ligeros. Una solución sintética de la condensación de combustible de etanol se ha formulado sobre la base de análisis químicos de muestras de soluciones naturales recogidos directamente desde el vehículo mientras se ejecuta durante su fase fría, es decir, dentro de los primeros 505 segundos. La evaluación de la resistencia a la corrosión de los aceros inoxidables ferríticos AISI 439 y 409, en comparación con el acero austenítico AISI 304, se llevó a cabo en presencia de una solución sintética de condensación combustible de etanol. El análisis comparativo de las curvas de polarización potenciodinámicas de los aceros estudiados indica que no hay diferencia en el comportamiento relativo a la corrosión de estos aceros. Además, se pudo observar ningún tipo de identificación de un potencial de picadura dentro de estas curvas. Tras la aplicación de las pruebas de Dip-seco, se puede comprobar que los aceros no, de hecho, tienen un comportamiento similar en cuanto a que no se sometieron a la corrosión, solamente la oxidación. Por tanto, podría llegarse a la conclusión de que todos los aceros inoxidables estudiados pueden ser recomendados para su uso en el silenciador trasero de los vehículos ligeros y que la elección de un acero inoxidable ferrítico sería la más económica entre los tres aceros estudiados. |
Due to the constant growth of the light vehicle fleet, great efforts have been made to reduce environmental damage caused by these vehicles with respect to reduction of emissions pollutants and reduce consumption of fossil fuels. In this way, several alternative fuels have being developed, such as ethanol in Brazil, based on cane sugar alcohol as a clean fuel source is widely spread and used (SCHINDELHOLZ,; RISTEEN, KELLY, 2014).
This study aims to characterize the behavior of ferritic stainless steels AISI 409 and 439, compared to austenitic stainless steel AISI 304, for corrosion occurring in silent rear light vehicle exhaust system-powered ethanol due to condensate formation.
Stainless steels are ferrous alloys of low-carbon, resistant to corrosion and oxidation and have at least 12% chromium in its chemical composition ( PECKNER,; BERNSTEIN,1977).
Stainless steels are those that exhibit greater corrosion resistance when submitted for an environment or aggressive agents, as compared to other classes of steels; also presents greater resistance to oxidation at high temperatures and in this case are called refractory steels (CONRADO ; BOCCHI; ROCHA FILHOAND BIAGGIO, 2003).
The higher corrosion resistance and oxidation of this steel type is mainly due to the presence of chromium-CA, from a certain value, in contact with oxygen, forms a film on the steel surface, which is waterproof and insoluble in the usual corrosive media. In simple terms, it means that the passivity in special circumstances a metal or metal alloy loses its chemical reactivity and behaves as an inert metal (PECKNER; BERNSTEIN, 1977).
The film, known as passive layer, resulting from the reaction between the material and the water present in the environment (air humidity condensed on the cold surface of the material). The reaction product is a stable oxy-hydroxide of chromium and iron, wherein the region closest to the metal surface and an oxide predominates in the region closest to the environment prevails hydroxide. Although invisible and very thin thickness, this film is extremely adherent to stainless steel and has a corrosion resistance increased as more chromium is added to the alloy (CARBO, 2003).
Corrosion resistance of stainless steels depends primarily on the chemical composition [19]. Generally, it can be said that the austenitic stainless steel is the most corrosion resistant and less resistant the martensítics. It is known however that the properties of "non-rusting" are associated with the existence of the passivation film that covers the surface of stainless steel. That way when evaluating the corrosion resistance of stainless steels, the condition of the passivation layer is as important as the influence of alloying (MANN, 2009).
Ferrite stainless steel, with cheap prices and low thermal expansion coefficients due to no addition of nickel, is excellent in corrosion properties and high temperature corrosion resistance compared with cheap prices, widely used in automobile industry. However, intergranular corrosion and sensitization become problems. To prevent these problems, stabilizing elements are added to improve corrosion resistance. 3 kinds of welding wire, where adjusted Ti contents of a stabilizing element were added to AISI 436 stainless steel, to perform FCAW (Flux Cored Arc Welding) and thus to evaluate microstructures and corrosive properties. The specimen having no Ti additive was transformed into Ferrite + Martensite structure, while the specimen having Ti additives into fully ferrite structure. As Ti contents increased, the structure became refined. The evaluation of corrosive properties revealed that the addition of Ti allowed pitting potential to increase and passive region to widen. As Ti contents increased, the difference between pitting potentials was very slight, and pitting size and degree decreased elements (HONG ; HAE , 2014).
It is a type of localized corrosion of electrochemical nature. Simply, it occurs when an alloy or metal, on contact with a solution containing chloride ions and other halide, suffers an attack forming cavities on its surface. These cavities begin with core size of two to three atoms [5]. Although affecting only small metallic surfaces parts, can cause rapid loss of thickness of metallic material, yielding pierces and points of stress concentration, leading to decreased mechanical strength of the material and the possibility of fracture (GENTLE, 1996).
Stainless steel is widely used in industry because of its good mechanical properties, good processability and excellent corrosion resistance. But it always suffers from pitting corrosion in a range of aggressive environments. It was found that the most commonly aggressive ion is the chloride ion in many natural and industrial environments.
Pitting is generally focused on a small area of metal surface. It always causes the device perforation and initiates stress corrosion cracks. Pits are nucleated at the microscopic scale and always covered by corrosion products, thus pitting is one of the more destructive and undetectable forms of corrosion in metals. In order to predict and prevent pitting corrosion, the dynamics of pit growth are worth further research. One mainly studies the changing rules of physical properties such as pitting sensitivity by electrochemical and acoustic emission methods. The parameters of pitting sensitivity include pitting potential, pitting initiation time, critical pitting temperature and so on; these parameters can indicate the pitting occurrence tendency in thermodynamics (TIAN, LI , DU, YE , 2015).
Among the stainless steels, austenitic family has the highest resistance solid-naked high temperature oxidation. However, for high temperature cyclic oxidation, stainless steel austenitic have an inferior behavior and are most cost them too and for this reason, we invest in the fact that ferritic stainless steels, well below cost to austenitic may fulfill the role of replacing traditional materials [8] . The replacement of traditional materials such as stainless steel carbon steel would like extra advantage weight vehicles reduction, because of the smaller wall thicknesses of stainless steel fabricated parts, and contributing to lower fuel consumption. In relation to the exhaust manifold, the weight reduction is about 40% (KIM; JANG ; WOO; KIM.; KIM, 2015).
The morphology of atmospheric pitting corrosion in 304L stainless steel plate was analyzed using MgCl2 droplets in relation to changes in relative humidity (RH) and chloride deposition density (CDD). It was found that highly reproducible morphologies occur that are distinct at different RH. Pitting at higher concentrations, i.e. lower RH, resulted in satellite pits forming around the perimeter of wide shallow dish regions. At higher RH, these satellite pits did not form and instead spiral attack into the shallow region was observed. Increasing CDD at saturation resulted in a very broad-mouthed pitting attack within the shallow dish region. Large data sets were used to find trends in pit size and morphology in what is essentially a heterogeneous alloy. Electrochemical experiments on 304 stainless steel wires in highly saturated solutions showed that the passive current density increased significantly above 3 M MgCl2 and the breakdown pitting potential dropped as the concentration increased. It is proposed that the shallow dish regions grow via enhanced dissolution of the passive film, whereas satellite pits and a spiral attack take place with active dissolution of bare metal surfaces (STREET ; ANGUS; COOK, 2015).
The "dip-Dry" is a test to simulate the conditions under which the materials are subjected in a vehicle exhaust system, i.e. heating cycles and pre-cooling feel during operation. Therefore allows evaluating the corrosion resistance of the materials in the presence of condensate formed during the surface cooling.
Dip and dry corrosion tests were carried out on Alloy A3003 (UNS A93003) using hydrochloric acid (HCl), acetic acid (CH3COOH), and a mixed solution. After 50 corrosion test cycles with the HCl solution, pitting corrosion occurred in some parts at higher concentrations, but corrosion weight loss was very low. In the case of the CH3COOH solution, general corrosion occurred, thickness decreased, and weight loss significantly increased as the concentration grew. In the case of the mixed solution, both general corrosion and pitting corrosion occurred, and corrosion mass loss increased as the HCl concentration grew. In addition, in concentrations of more than 50 ppm of HCl, the number of pits significantly increased. Electrochemical polarization measurements were done to understand the corrosion forms for each acid. In the electrochemical polarization, the pitting potential became less noble with increasing concentration of HCl. No distinct effect of CH3COOH on the pitting potential was confirmed, but corrosion current density increased with increasing concentration of CH3COOH. For the mixed solution, both of these effects were confirmed. These electrochemical polarization results correspond well with the dip and dry corrosion test results (IWAO; YOSHINO; EDO; SHU, 2015).
In a study conducted by Dos Santos (2008) stainless steel substrates of exhaust mufflers made of aluminized stainless steel can be exposed to solutions containing Al3+ ions long after the dissolution of their Al coating. This study examined the corrosion behavior of stainless steel in synthetic condensed water that contained different amounts of Al dissolution. The corrosion resistance of stainless steel in the solution containing dissolved Al3+ ions decreased. The unstable
passive film contained Al oxides (or hydroxides), which decreased the protective properties of the stainless steel. The dissolved Al3+ ions in the exhaust condensed solution had a negative effect on the corrosion resistance of the stainless steel.
A systematic study was carried out by Lou; Yang ;Singh (2009) to understand the effect of ethanol chemistry (chloride, water, pHe, and oxygen level) as well as other controlling parameters on the stress corrosion cracking (SCC) behavior of X-65 carbon steel in simulated fuel-grade ethanol (SFGE). The SCC susceptibility of carbon steel was evaluated using the slow strain rate testing (SSRT) method. Chlorides strongly affect the SCC initiation and growth, and a higher concentration of chloride leads to a higher crack density and velocity. The addition of water in the ethanol influences the surface passivation in SFGE. SCC-to-pitting corrosion transition was observed above 2.5% water concentration in SFGE. pHe was also found to be a critical factor influencing the SCC susceptibility with alkaline SFGE inhibiting SCC initiation in carbon steel. Strain rate affected the SCC behavior of carbon steel, where slower strain rate caused a larger crack length and a higher crack density, but a lower crack velocity. Hard inclusions (alumina and silicate) in X-65 steel acted as early crack initiation sites due to the higher local plastic deformation that occurred near the inclusions. By adjusting the ethanol chemistry, SCC susceptibility of a commercial fuel-grade ethanol can be mitigated.
The set of irregularities, i.e., small protrusions and recesses featuring a surface. These irregularities can be assessed with electronic devices, the Surface Roughness (TESTER SURFACE ROUGHNESS. ELECTRONIC HANDOUT, 2009).
The steels studied in this research were ferritic AISI 409 and 439 compared to austenitic stainless steels AISI 304 were supplied by ArcelorMittal Inox- Brazil in the plates form drawn after the final stage of cold rolling, final annealing and pickling, i.e., with surface finish called 2D and thicknesses of 0.5 mm. Two steel 409 chemical compositions were used, identified by 409A and 409H. The chemical composition of steel is given in Table.1. Two chemical compositions of the steel used were 409, indicated by 409A and 409H. The difference in composition is the variation is small fraction of the stabilizers that objective a greater steel stabilization, in the
case 409H.
Table 1- Chemical composition (mass%) of stainless steels studied [15].
Steel |
C |
Mn |
Si |
P |
S |
Cr |
Ni |
Mo |
Ti |
Nb |
*N2 |
*C + N |
Stabilization |
AISI 304 |
0,044 |
1,11 |
0,45 |
0,028 |
0,0030 |
18,21 |
8,03 |
0,055 |
0,00 |
0,01 |
471 |
916 |
- |
AISI 439 |
0,010 |
0,16 |
0,39 |
0,031 |
0,0008 |
17,43 |
0,18 |
0,047 |
0,19 |
0,22 |
109 |
211 |
19,4** |
AISI 409A |
0,007 |
0,17 |
0,45 |
0,020 |
0,0004 |
11,22 |
0,15 |
0,013 |
0,14 |
0,01 |
85 |
159 |
8,9*** |
AISI 409H |
0,006 |
0,15 |
0,39 |
0,024 |
0,0025 |
11,31 |
0,17 |
0,022 |
0,17 |
0,01 |
72 |
141 |
12,1*** |
(**)Fraction stabilizer (Ti+Nb)/(C+N) - (***)Fraction stabilizer Ti/(C+N) - (*) in mg/L.
1x1cm sample of stainless steels plates studied were prepared metallographically by conventional method. The specimens were embedded in the rolling plane, which surface was subjected to the same corrosion tests. The polishing involving sanding following 600, 800 and 1500 mesh, rotating the specimens 90 and in the washing with running water between the stages of sanding; followed by mechanical polishing with diamond paste cloths 3 and 1μm for about 30 minutes each and polishing colloidal silica suspension 0,04μm - OP-S - for final polishing by five minutes. Chemical attacks to observe the microstructure present in the specimens prior to the corrosion testing and grain size of the second ASTM E 29112-96 with Kaling`s reagent or Villela for specimens of AISI 409 (for 15 seconds) and reagent aqua regia to AISI 439 specimens (by 1 minute) and 304 (during 1 minute and 20 seconds).
In the AISI 304 electropolished sample was carried out with perchloric acid for 5 seconds, 0.5A current and voltage of 1V prior to etching in order to remove surface deformation that may provide austenitic microstructure after the sanding step.
For the collection of vehicle fuel, alcohol condensate tests were carried up following the NBR 6601 standards only covers the first part of the cold phase called cycle, which is equivalent to the first 505/2 of vehicle operation. Experiments for gathering and analysis of alcohol condensate were performed at Fiat Power Train Emissions Laboratory, Fiat Group Company responsible for manufacturing the engines of the brand's vehicles and certified by the competent bodies.
The gathering of the condensate was made using a condenser system attached to the exhaust pipe of a vehicle with engine 1.0 Flex (powered by alcohol and / or petrol) fueled only with alcohol that sucked the gases and the produced condensate and diretioned to condensate collection container. The gathering of natural condensed followed the steps below:
1. Assembly of a condensing system coupled to unloading the vehicle;
2. Departure for operation of the vehicle idling;
3. Staying vehicle idling during 505/2;
4. De-linking of the vehicle;
5. Container gathering with natural condensate produced during 505 seconds operation of the vehicle;
6. Storage and transmission of specimens taken for chemical analysis.
The average volume of condensate produced in each collection was 200 ml. From this volume, 30 ml was used to unburned alcohol analysis and aldehydes which were held in Emissions Laboratory FPT - Fiat motor factory of SA, equal volume for analysis of anions held at the Environmental Measurements Laboratory CETECMG.
They were raised in stainless steel specimens AISI 304, 439 and 409A in a potentiostat / galvanostat Autolab PGSTAT 20, Eco Chemie data collected from system program. Connected to this system a conventional electrochemical cell provided with three entered was connected to the system, the first entry for the working electrode represented by stainless steel sample to be tested whose area exposed to the electrolyte was 1,0cm2, the second at calomel reference electrode saturated with salt bridge 1M KNO3 and the third to the counter electrode consists of a platinum wire.
Assays were performed using as he-trinitrotoluene synthetic freshly prepared solution of condensed alcohol previously degassed for 30 minutes with nitrogen gas at room temperature, by varying the potential of -0.6 to 1.5 V and at a rate of scan 0,167mV / s [16]. To ensure the repeatability of the data were raised four curves of each steel studied trials for lifting of polarization curves were realized in Metallurgical Technology Sector IEF-MG. - Brazil.
Specimens for these assays were cut in dimensions 10x10mm, welded to a metal wire, which makes the connection between the sample and the working electrode for passing a current, and then embedded in cold-cure resin. After this step, the specimens were ground in sequence 240, 320, 400 and 600 mesh by taking care to rotate the sample 90 and wash with water between steps of sanding. After the grinding step, washed with detergent and cotton, dried with cold air jets and placed in a desiccator for about 24 hours prior to testing.
The electrolyte was compared to the exposure surface lamination. The welding process used to connections was capacitive welding so that the sample not heat up this process. It was necessary to use a calomel reference electrode immersed in a saline solution instead of 1M KNO3 and KCl is more common.
From the polarization curves were obtained the electrochemical parameters of corrosion potential (Ecorr), corrosion current (Icorr) and resistance polarization (Rp) by taking the average of each of the four values.
A cycle that simulates the most aggressive condition of the vehicle, i.e. stopping shooting cycle and with small distances was utilized to evaluate the possible steels to be used in the system exhaustion.
This cycle, referred to herein as 'urban cycle' consists of the following steps (DI CUNTO; JULIO CESAR, 2005)
1. Immersion for 5 minutes in synthetic condensate solution to 50 ° C, followed by resting in air for 5 minutes, repeated for 10 hours, which characterizes the "Dip" cycle.
2. After the cycle "Dip" is the beginning of the cycle "Dry" where the specimens are in an oven at 300 ° C for 1 hour, then 1 hour cooling. Testing cycled type "Dip-Dry" for a period of 500 and 1000h, which comprised 41 and 83 cycles of immersion / emersion, were performed respectively. Care was taken up to exchange the synthetic solution of alcohol condensed 100 hours each test in order to avoid loss of solution characteristics due to loss of volatiles. After the tests, the first evaluates was performed visual inspection of the sample.
For the tests of "Dip-Dry" were used specimens of stainless steel AISI 304, 439, 409A and 409H with dimensions of 25x190mm and finishing 2D which is a name of manufacturer indicating that the material was cold rolled, annealed and pickled. This finish was chosen because steel is used in the cold part of the vehicle exhaust system. Tests on "Dip-Dry" were held in ArcelorMittal Inox Brazil, Timoteo – MG, Brazil.
Microstructural characterization
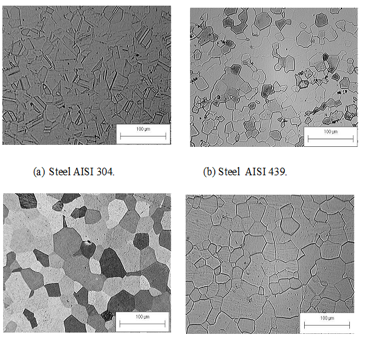
The Figure 1 shows the microstructures of steels studied in the state as received. It is observed in AISI 304 steel an austenitic matrix with presence of twinning and AISI 439 steel and 409 a ferritic matrix with the occurrence of precipitates rich in Ti and Nb.
Figure 1 - Typical microstructure of steels.
(a) AISI 304 electrolytic polishing and attack with royal water, grain size 8.7 ASTM.
(b) AISI 439 mechanical polishing and attack with royal water, grain 8.7 ASTM.
(c) AISI409A mechanical polishing and attack Kaling's, grain 6.6 ASTM.
(d) AISI 409H mechanical polishing and attack Villela, grain 7.5 ASTM.
Surface roughness
The surface roughness of specimens of stainless steels studied here was measured before and after the tests "Dip-Dry" 500 and 1000 hours in order to evaluate the degradation of exposed surfaces specimens to synthetic fuel alcohol solution. The parameters measured were the Ra and Rz by a Hommelwerke rugosimeter - T1000.
Brightness and color Brightness
To measure the brightness of the surfaces of the steel specimens before and after the test "Dip-Dry" by a gloss meter Byk Gardner - Mirror Gloss and geometry of 60 °.
Byk Gardner color spectrophotometer was used Spectro-Guide Sphere gloss for measuring the color variation suffered by stainless steels subjected to testing "Dip-Dry".
Mass and thickness
For mass measurements before and after the tests of "Dip-Dry" was used an analytical balance Mettler-AE200. To determine the thickness varying thicknesses of the specimens before and after test "Dip-Dry" was used a digital micrometer Mitutoyo.
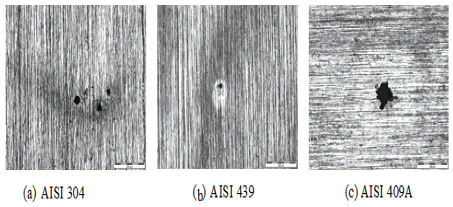
Optical microscopy was used to characterize the microstructure of each steel and studied for surface characterization of the specimens before and after corrosion tests to identify particular aspects of each steel after each corrosion test. In the micrographs of 2 it is noted that the pits are larger occurred on the stainless steel surface AISI 304 and 409 A and smaller in AISI 439.
Figure 2. Micrographs of stainless steels after polarization tests
in synthetic solution condensate contaminated fuel alcohol
with chloride ions, indicating the presence of pits.
(a) AISI 304 (b) AISI 439 (c) AISI 409A.
The table 2 shows the chemical composition of synthetic solution of ethanol condensate produced from the chemical analysis of several specimens of natural condensate collected from a vehicle powered by ethanol.
Table 2 - Chemical composition of synthetic solution of ethanol
condensed, average conductivity 5,0μs / cm and pH average of 5.97.
Type |
Amount (mg/L) |
Na+ |
0,35 |
K+ |
0,444 |
Ca++ |
0,16 |
NO2- |
0,7 |
SO4-- |
0,526 |
Cl- |
0,28 |
ANB |
1,00 |
ANB – alcohol not burned.
Table 3 - Electrochemical parameters obtained from the test polarization
curve of stainless steel AISI 304, 439, and 409A in synthetic ethanol solution
and condensed at ambient temperature (*).
Specimens |
Ecorr (V) |
Icorr (A/cm²) |
Rp (Ω) |
AISI 304 |
-0,233±0,030 |
-9,463±0,218 |
1,48E+0,5±606363,7 |
AISI 439 |
-0,250±0,045 |
-9,049±0,697 |
7,16 E+05±242648,3 |
AISI 409A |
-0,218±0,044 |
-9197±0,166 |
9,23E+05±153969,0 |
(*)Mean ± standard deviation in each column.
Comparatively analyzing the curves does not observe significant differences in the behavior of three steels in respect to corrosion in synthetic fuel alcohol solution at room temperature. This behavior may possibly be attributed to the low aggressiveness of the solution. Polarization curves already raised in synthetic solution gas condensate those same three steels indicate a lower behavior of these steels compared with curves raised in synthetic solution of ethanol condensate which indicates that the condensed gas is more damaging to the system exhaust of vehicles that fuel alcohol condensate.
In synthetic gas condensate solution all stainless steels studied showed that there is a current increase in potential of the order of 700mV and that 439 and 409A AISI steels showed a "secondary passivation" in potential of about 800mV, followed by the reaction of oxygen evolution, approximately 1200mV.
This reduction current was not observed for stainless AISI 304. Precipitation and deposition of corrosion products may have been responsible for the reduction of the current (DI CUNTO; JULIO CESAR, 2005). It should further be considered that the solution of the synthetic condensate alcohol to be much diluted has a high aggressiveness. Failure compensation ohmic drop due to the resistivity of the electrolyte, often called IR drop may cause distortions in the polarization curves (WOLYNEC, 2002).
Essay "Dip-Dry" Figures 3 and 4 shows the specimens of stainless steel AISI 409H, 409A, 439 and 304 after completion of the tests "Dip-Dry" lasting 500 and 1000 hours respectively.
Figure 3 - Test specimens after 500 hours of test "Dip-Dry"

Figure 4 - Test pieces after 1000 hours of testing "Dip-Dry".

On visual inspection, it was found that the steels showed no corrosion on their surfaces, but suffer oxidation, which is evidenced by the color difference in some areas of different specimens both tested up to 500 to 1000 hours as in hours. AISI 409A suffered the most oxidation followed by AISI 409H, stainless steels AISI 439 and 304 showed similar superficial aspect each other and higher than the other two steels. Probably the result of increased oxidation of AISI 409A and 409H steels are due to the lowest chromium content in relation to AISI steels 304 and 439. It is also observed that the interface of the specimens, air and condensate the same did not happen when the condensateused is ethanol, it is known that when using the condensed gas that attack is intense confirming that the condensed gas is most damaging to the exhaust system of vehicles to the fuel alcohol condensed (DI CUNTO; JULIO CESAR, 2005).
The specimens surfaces tested, the polarization curves were evaluated by micro-optics copies before and after the test to observe possible surface defects due to the mechanical grinding that could be potential regions pitting formation , as color post-test with surface defects are more likely to pitting nuclear [5] .
There was no difference observed on the sample surface before and after the polarization curve of tests which confirm the results obtained from the evaluation of polarization curves.
Test "Dip-Dry" Surface roughness, brightness, color, mass and thickness of the specimens were evaluated before the start of the test and after the tests of 500 and 1000 hours. The tables 4, 5, 6 and 7 show the roughness values; brightness and color; mass and thickness measure of before and after the test "Dip-Dry".
Table 4- Initial roughness values and final of stainless steels after testing "Dip-Dry" 500 and 1000 hours.
|
Initial |
Final |
||
AISI Steels |
Ra (µm) |
Rz (µm) |
Ra (µm) |
Rz (µm) |
409H 500h |
0,457 |
3,39 |
0,477 |
3,45 |
409H 1000h |
0,428 |
3,13 |
0,497 |
3,33 |
409A 500h |
0,205 |
2,13 |
0,250 |
1,68 |
409A 1000h |
0,323 |
4,40 |
0,235 |
1,67 |
439 500h |
0,256 |
2,18 |
0,213 |
1,88 |
439 1000h |
0,216 |
1,83 |
0,207 |
1,90 |
304 500h |
0,080 |
0,76 |
0,090 |
0,87 |
304 1000h |
0,080 |
0,87 |
0,106 |
0,93 |
Table 5. Change in color values of stainless steels after testing "Dip-Dry" 500 and 1000Hours.
|
||||
Table 6. Values of brightness and color early and late stainless steels after testing "Dip-Dry" 500 and 1000 hours.
|
||||||||
|
Steel |
||||||||
Table 7. Mass values and initial and final thickness of stainless steels after testing "Dip-Dry" 500 and 1000 hours.
|
Initial |
Final |
||
AISI Steel |
Mass (g) |
Thickness (mm) |
Mass (g) |
Thickness (mm) |
409H 500h |
17,5034 |
0,503 |
17,5024 |
0,507 |
409H 1000h |
17,4916 |
0,504 |
17,4909 |
0,511 |
409A 500h |
17,0820 |
0,495 |
17,0809 |
0,498 |
409A 1000h |
17,0922 |
0,493 |
17,0915 |
0,501 |
439-500h |
16,7634 |
0,478 |
16,7630 |
0,488 |
439 1000h |
16,7349 |
0,479 |
16,7342 |
0,484 |
304-500h |
17,1645 |
0,480 |
17,1642 |
0,487 |
304 1000h |
17,0743 |
0,483 |
17,0742 |
0,492 |
According to the presented in Tables 4, 5, 6 and 7 it is concluded that there was no large variation of the masses and thicknesses of the specimens, what is observed is a slight variation of surface roughness of stainless steel AISI 409A and 409H and the variation of the brightness and color of the specimens of stainless steels, which proves the oxidation of the surfaces of the specimens and this more visible variation in stainless steel specimen AISI 409A followed by AISI 409H.
The AISI 439 and 304 steels showed similar behavior to each other and superior to the other two tested steels which suggests that they are the best to be used in the coldest part of the exhaust system of vehicles powered by fuel alcohol is desired to apply a material with greater durability. Nevertheless one can consider all stainless steels studied here as suitable for use in the cold part of the vehicle exhaust system moved by alcohol fuel.
The raised potentiodynamic polarization curves for the three types of tested steels, AISI 304, 439 and 409 indicate similar behavior in corrosion resistance in synthetic condensate ethanol solution.
However, these curves must be used only for benchmarking between the studied steels. The electrochemical parameters obtained do not represent actual values due to high ohmic resistance of synthetic solution of ethanol condensed, next to the distilled water.
The evaluated stainless steels did not show pits on their surfaces when performing the polarization curves and the essay "Dip-Dry".
None of the steels evaluated in the essay "Dip-Dry" suffered corrosion process; it was observed so mind-oxidation of the surfaces of the specimens.
Stainless AISI 409A is presented the worst performance in the essay "Dip-Dry" followed by AISI 409H.
Stainless steel AISI 439 and AISI 304 showed similar behavior in the "Dip-Dry" experimental.
Essay "Dip-Dry" is observed that stainless AISI 409H is slightly higher than AISI 409A; This feature can be attributed to the stabilizers fraction difference The higher stability means more carbon combined with Ti, lower level of chromium carbides and therefore more free chromium to increase the corrosion resistance of passive layer.
The ferritic stainless steels studied in this research can be specified for use in the cold part of the vehicle exhaust system powered by ethanol.
The fuel alcohol condensate is less aggressive cold part of the vehicle exhaust system that the condensed gas, as evidenced by comparing the literature.
Our special thanks to the Emissions Laboratory FPT - Fiat motor factory, at the Environmental Measurements Laboratory CETECMG and also to the Metallurgical Technology Sector IEF-MG. - Brazil.
ASTM G5 – 94 Standard Reference Test Method for Making Potentiostatic and Potentiodynamic Anodic Polarization Measurements. Sept. 1997.
ArcelorMittal Inox Brazil - Corrosion Laboratory of Surface, August, Timothy – MG .2009.
Azmat,N.: S. ;K., Ralston,D.; T. Muster; B. Muddle, C. and I. Cole, S. A HighThroughput Test Methodology for Atmospheric Corrosion Studies, Electrochem. Solid-State Lett., 14(6), C9–C11. 2011.
Carbo, H, , M. Stainless Steels - Application and Specification. São Paulo: Core Inox.2001.
Conrado, R. ; N. Bocchi; R. Rocha Filho,C. and S. Biaggio,R. Corrosion resistance of colored films grown on stainless steel by the alternating potencial pulse method, Electrochemica Acta, 48, p.2417-2424. 2003.
De Oliveira,G. ; H. L.; N. Falleiros, A. Potential of the determination of Stainless Steel Ferritic Pitting by potentiodynamic and potentiostatic methods. Production Scientific Initiation (Department of Metallurgical and Materials Engineering of EPUSP materials) – EPUSP. 2002.
Di Cunto, C. Julio Cesar. Study of Resistance Stainless Steel Corrosion for Use in Cold Part of Vehicle Exhaust Systems. 2005. Dissertation (Master in Science in Nuclear Technology. Area - Materials) - Institute of Energy and Nuclear Research, São Paulo. 2005.
Gentle, V.. Corrosion. Rio de Janeiro: Technical Books and Scientific Publishing. 1996.
Hong, Y; T. S. , W. Hae L. Characterization of Corrosion Resistance in a Ferritic Stainless Steel Stabilized with Ti Addition Ja young, Int. J. Electrochem. Sci., (9)7325 – 7334. 2014.
Iwao, S.; M. Yoshino, M. Edo, and K. Shu . Behavior of Alloy A3003 After Brazing in HCl, CH3COOH, and Mixed Solutions. Corrosion: May, 71(5), pp. 598-605. 2015.
Kim, M. , J. ; S. Jang, I. ; S. Woo, H.; J. Kim, K.; and Y. Kim, H. Corrosion Resistance of Ferritic Stainless Steel in Exhaust Condensed Water Containing Aluminum Cations. Corrosion: March 71(3), pp. 285-291. 2015.
Lou, X., D. Yang, and P. M. Singh Effect of Ethanol Chemistry on Stress Corrosion Cracking of Carbon Steel in Fuel-Grade Ethanol. Corrosion: December 2009, 65 (12), pp. 785-797. 2009.
Mansfeld, F; S. TSAI. Corrosion kinetics in low conductivity media it iron and nickel in ethanolic HCl, Corrosion Science, 27 (8), p. 873-877. 1987.
Mann, B S. Erosion visualization and characteristic of a two dimensional diffusion treated martensític stainless steel hydrofoil. Wear 1998;217:56–61.2009.
Peckner, D; I. Bernstein, M. Handbook of Stainless Steels. United States of America: McGraw-Hill Book Company. 1977.
Surface Roughness. Electronic handout PUC - Rio _ Digital Certification No. 0016226 / CA, consulted in September. 2009.
Street, R. S.; N. Angus, M. J. M. C.; H. Cook, B.; L. Mohammed Ali, L.; G. Trevor ; J. Alison; J. Davenport. Faraday Discuss., 180, 251–265 The Royal Society of Chemistry. 2015.
Schindelholz, E. ; B. Risteen, E. and R. Kelly, G. Effect of Relative Humidity on Corrosion of Steel under Sea Salt Aerosol Proxies, J. Electrochem. Soc., 161(10), C460–C470. 2014.
Schindelholz, E; B. Risteen, E. and R. Kelly, G. Effect of Relative Humidity on Corrosion of Steel under Sea Salt Aerosol Proxies, J. Electrochem. Soc., 161(10), C450–C459. 2014.
Tian, W. , Yi. Ai , S. Li , N. Du , C. Ye Pitting Kinetics of 304 Stainless Steel Using ESPI.
Wolynec, S. Electrochemical Techniques for Corrosion. São Paulo: EDUSP.2002.
1. Cetec - Fundação Centro Tecnológico de Minas Gerais. Avenida José Cândido da Silveira, 2000 – Horto- Belo Horizonte - MG- Brazil. paulasprates@yahoo.com.br
2. UNESP. Universidade Estadual Paulista. Rua Geraldo Alckmin, 519. Itapeva. SP. Brazil. glaucia@itapeva.unesp.br
3. UNESP. Universidade Estadual Paulista. Rua Geraldo Alckmin, 519. Itapeva. SP. Brazil. caraschi@itapeva.unesp.br
4. USP Universidade de São Paulo. Faculdade de Ciencias Farmaceuticas de Ribeirao Preto, Brazil , wasim.syed@usp.br