

Vol. 38 (Nº 12) Año 2017. Pág. 1
Marcelo Gorri MAZZALI 1; Ieda Kanashiro MAKIYA 2; Francisco Ignácio Giocondo CESAR 3
Recibido: 20/09/16 • Aprobado: 18/10/2016
3. Qualification and Validation
ABSTRACT: The pharmaceutical logistics in Brazil, which is growing year after year, the main modal shift this burden is road transport, care in the conservation of pharmaceutical products during transport process is critical because it incorporates the principles of safety and therapeutic efficacy, essential for a pharmaceutical product is suitable for human consumption. In Brazil there are still issues regarding legislation, costs, liabilities, lack of specialized transportation in the cold chain, there is no requirement that shipping should be made with the qualitative criteria of the load, there is only mentions in relation to maintain the same quality during transportation, for these reasons end load being carried as normal load. It is an exploratory, descriptive and analytical study, with qualitative and quantitative approach to the profile of the pharmacist in the logistics of drugs through a survey conducted with several companies that cover part or all of your supply chain involving transport, storage, cross-docking, picking, handling of cargo, and this study in Campinas-SP region. The methodology used was descriptive study through data collection directly on the carriers through the technical officer using as an information tool and a standard search after the questionnaire response, technical visit in the company. As it is a little explored area and streamlined in Brazil, this study also evaluates the role and pharmacist acting in this way take steps so that the pharmacist to act more as a manager than just a doer of legislation and regulatory documents, ensuring the product characteristics. |
RESUMO: A logística farmacêutica no Brasil, que está crescendo ano após ano, o principal modal deslocar este fardo é transporte rodoviário, cuidados na conservação de produtos farmacêuticos durante o processo de transporte são crítico porque incorpora os princípios da segurança e eficácia terapêutica, essenciais para um produto farmacêutico é apropriado para consumo humano. No Brasil existem ainda questões relativas à legislação, responsabilidades, custos, falta de transporte especializado na cadeia de frio, não há nenhuma exigência de que o transporte deve ser feita com os critérios qualitativos da carga, há apenas menciona em relação ao manter a mesma qualidade durante o transporte, para acabam com estas razões carga transportada como carga normal. É um estudo exploratório, descritivo e analítico, com abordagem qualitativa e quantitativa para o perfil do farmacêutico na logística de drogas através de uma pesquisa realizada com diversas companhias que cobrem parte ou a totalidade de sua cadeia de suprimentos, envolvendo o transporte, armazenamento, cross-docking, picking, movimentação da carga e este estudo na região de Campinas-SP. A metodologia utilizada foi o estudo descritivo através de coleta de dados diretamente sobre as transportadoras através do oficial técnico usando como uma ferramenta de informação e uma busca padrão após a resposta do questionário, técnica visitar na empresa. Como é uma pequena área explorada e simplificada no Brasil, este estudo também avalia a função e o farmacêutico agindo em passos de tomar este caminho, então que o farmacêutico para atuar mais como um Gerenciador do que apenas um executor de legislação e documentos normativos, garantindo as características do produto. |
The pharmacist profession has been changing along time, like many other professions. To consider the wide range of health services and actions, one must also think about the actions and services of Pharmaceutical Assistance. Considering that most interventions in health involves the use of medications and that this use can determine the results, the Pharmaceutical Assistance must be considered as a whole.
Full attention to health services can’t be delivered when the Pharmaceutical Assistance is reduced to medicine logistics only (acquire, store and distribute). It is necessary to aggregate value to health actions and services by developing the Pharmaceutical Assistance. To do so, it is necessary to integrate Pharmaceutical Assistance in the logistic system, count on trained professionals, program the operation systems correctly, have space and flux according to logistic demands to supply the market, the capacity to store, adequately distribute and transport the pharmaceutical product, manage inventories, deliver products and to monitor all the process of the logistic supply chain.
This work demonstrates the current state of the logistic market in the São Paulo region, Brazil. We aim to demonstrate the role of the pharmacists based on the following questions: Does this professional have specific training for logistics? How long does he/she work in this company? How long does it take to develop a management project? Does the pharmacist understand pharmaceutical management or believes that this service applies only to those that work in drugstores.
The pharmacists is key in the logistic segment, since he is no longer a mere professional abiding to the legislation and becomes a link to other segments, improving process, integrating teams, improving human resources and the knowledge of pharmaceutical products.
The pharmacist manager in logistics is essential for the company success. Besides, this professional guarantees the process that allows the final product to reach the consumer with total quality, thus maintaining its medical effects. The logistic quality in the pharmaceutical supply chain reduces the chances of adverse effects, intoxication and side effects.
The term “Pharmaceutical Attention” has been adopted and officialized in Brazil after discussions led by the Pan American Health Organizations (OPAS), World Health Organization (WHO), Health Ministry (HM) from Brasil, among others. In this meeting, Pharmaceutical Attention was defined as “a model of pharmaceutical practice, developed in the context of Pharmaceutical Assistance. It comprehends actions, ethical values, behaviors, skills, commitment and co-responsibility in preventing diseases, and health promotion and recovery; all integrated to the health team. It is the direct integration of the pharmaceutical and the user, aiming at the rational pharmacotherapy and the acquisition of measurable and pre-defined results, in order to achieve better life quality. This interaction also must include the conceptions of its subjects, respecting their biopsycosocial uniqueness under the scope of integrating health actions (T.N.: free translation).” [11, 19, 22]
Besides the concept of Pharmaceutical Attention, the same meeting mentioned above defined the macro components of the professional practice of Pharmaceutical Attention, such as: health education (promoting rational use of medicines), pharmaceutical orientation, medicine delivery, pharmaceutical attendance, pharmaco-therapeutic monitoring and continuous activity record. [11, 19, 20, 21, 22]
However, the most commonly accepted definition of Pharmaceutical Attention used today by researchers is the one elaborated by Hepler and Strand (1990), where Pharmaceutical Attention is presented as part of the pharmaceutical practice that allows the interaction of the pharmaceutical with the patient, aiming to attend to the medical-related needs of the latter. [20, 21, 22]
Pharmaceutical Attention is the wide range of actions carried out by a pharmaceutical – in collaboration with other health professionals – that aim to promote the rational use of medications and the maintenance of the treatment’s effectivity and safety.
According to the WHO, Pharmaceutical Attention is: “a concept of professional practice in which the patient is the main beneficiary of the pharmacist’s actions. The Pharmaceutical Attention is a compendium of attitudes, behaviors, commitments, inquietudes, ethical values, functions, knowledge, responsibilities and skills of the pharmacist to carry out pharmacotherapy, aiming to obtain therapeutic results in the patient’s quality of life (T.N.: freely translated). [21, 22]
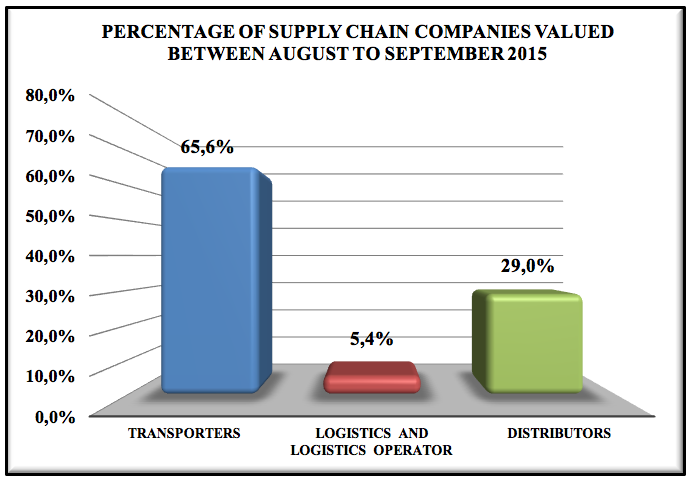
Figure 1 - Pharmaceutical logistics to the final consumer, developed by the author
2.2. Pharmaceutical assistance
Pharmaceutical Assistance is a concept that encompass the vast array of practices aimed to individual and collective health in the logistics cycle, with the medicine as the main input. The logistic cycle includes manufacture, acquisition, programming, storage, distribution and delivery). It is a multidisciplinary activity, but pharmacists are responsible for the rational use of medications. [11, 19, 20]
Resolution no 338 from 2004 of the Brazilian National Health Council states that pharmaceutical assistance is a set of actions aimed at the promotion, protection and recovery of both individual and collective health, using medications as the main input. This resolution promotes the access and rational use of medications. The set of actions mentioned before include the selection, programming, acquisition, distribution, delivery, quality assurance of products and services and monitoring of medication use; in order to obtain concrete results and improve population well-being.[1, 2, 3, 4]
This demands continuous articulation with technical sectors, management, health programs coordinations, family health programs, community health programs, sanitary regulation, epidemiology, financial, planning, inventory, licitation, audition, ministries, regulation agencies, health councils, health professionals, universities and other segments of the society to improve the action, divulgation and support for its actions. [11, 19, 20]
Pharmaceutical assistance is a dynamic and multidisciplinary process that aims to supply systems, programs and health services with high-quality medication. Thus, allowing patients to access the medication needed. The pharmacist is key to guarantee that those that need medications get access to it. It is worth noting that such access require a trained professional. Therefore, there is a demand to train managers and professionals in all clinical (prescription, delivery, management, monitoring and enrollment) and management (selection, programming, acquisition, storage and distribution) activities related to pharmaceutical assistance.
In most cases, medications are the therapeutic intervention with the best cost-effectiveness ratio, as long as they are prescribed and used rationally. On the other hand, regarding medication access, there is a clear inequality in medication consumption and demographic distribution, with 80% of all medications consumed by only 18% of the population in developed countries (North America, Europe and Japan). [11, 19,20, 21,22]
In the health service chain, pharmaceutical assistance is a strategic instrument and its actions must aim at medication access, quality and rational use; caring for the sustainability of the system. Professionals in this segment face the challenges for the qualification of aspects related to the development of clinical and management activities. The approval of the Brazilian Constitution in 1988 fomented the implementation of health policies that comprehend health activities and inputs that improve human well-being. [9, 10, 12, 13]
The Supply Chain represents a set of activities that include acquisition, storage, transformation, packaging, transportation, internal movements, distribution and all the support necessary to integrate and manage the total flux of a distribution channel to the final user. [9, 10, 12, 13]
In order to keep the quality of pharmaceutical products, all stages of the distribution chain should abide to legislation. All logistic activities of pharmaceutical products should be carried out according to the principles of good manufacturing practices (GMP), good storage practices (GSP), good distribution practices (GDP) and good transportation practices (GTP). [9, 13, 21,28,29]
The decision to outsource a portion of the stock allows the pharmaceutical industry to improve the use of industry space for production. This practice has been increasing the importance of the logistic operator, whom basically receives pharmaceutical products, stock them according to invoicing and dispatch them. These agents are investing in their own CD to reply to one or more clients. [13, 14, 17]
The transport logistics is a significant problem since it is a complex system that demands time, trained labor, planning, dimensioning of vehicles and location. [13, 14, 17]
During the transportation of pharmaceutical products, one of the most important factors is temperature. The internal condition of the vehicle, the number of items, distance and duration of transportation, load and unload, may cause loss of efficiency of the product, due to oscillation in temperature and moisture. [13, 14, 15, 17]
Management means administration in an institution, a company, a social enterprise that needs to be managed. The objective of management is growth, established by the company through organized human effort towards a defined objective. Institutions can be private, mixed economy societies or non-profit organizations.
Management rose after the industrial revolution, as professionals searched for solutions to new problems, using several scientific methods to administrate business. Thus, the science of management began, due to the use of models and administrative techniques. [19, 20,21]
Management belongs to the human sciences since it deals with groups of people, in order to maintain the synergy among then, the company structure and the available resources. The manager has the role to solve problems, organize financial and technological resources, communicate, lead and motivate people, make decisions and evaluate them and control all these elements. [18, 19]
As we all know it, the labor market is increasingly dynamic, competitive and challenging. For example, qualification demands are higher in all careers, requiring new competences and fast responses . Despite the knowledge that guides and centers most of our qualification, we are constantly facing new and unexpected challenges in the labor market. In all fields, the professional stumbles upon new domains. Another major issue of concern is the pragmatism of the professional work, defined by the competition and a sense of urgency: problems must be solved immediately and we are more than never bound to the customer’s choices. In other words, in the workplace, there is a balance of consequences and, therefore, we cannot be indifferent to these new knowledge demands. [18, 19,20]
Most of the activities of the services category is carried out by people and, in the case of pharmacies, we need qualified people more than never. In this segment we need people to attend clients with special characteristics, whose emotional state is not the same of a client that is buying a screw, a sandwich or any other product. In the pharmaceutical context, to deliver a product correctly it is too little if it is not followed by the adequate treatment orientations. [19, 20,21]
The logistic environment is not free from the challenges mentioned above, the pharmaceutical must orient, inform, train the operational team, set goals and be part of the technical-scientific team. Today, the pharmaceutical in the supply chain is only a complier of the legal demands. This professional is rarely seen managing a whole team or its branch. There is always somebody above him with a degree in management, marketing or logistics.
According to the World Health Organization (WHO, 2011), qualification is the act or effect of qualifying and attributing quality. Qualification is carried out through registered tests that demonstrate, with a high degree of certainty, that a given process will achieve its pre-determined acceptance criteria.
Validation is a “registered action testifying that any procedure, process, equipment, material, activity or system is indeed achieving the expected results” (T.N.: free translation). It is key for the Best Practices in pharmaceutical products logistical chain. [1, 2, 4, 5]
Validating the process is the main quality tool for controlling and monitoring product conservation. It aims at assuring that a given operation was achieved the expected result according to pre-defined acceptance criteria, besides supplying important information for product stability and key points for quality control and deviation. [1, 4, 7]
Considering quality; process safety and efficacy will be guaranteed as long as the recommendations of the sanitary authorities and the manufacturer are respected. [1, 3, 4, 7, 28]. Therefore, to guarantee that quality, efficacy and safety are not hindered during the logistic chain, validation must be carried out during all logistic stages: from the manufacturing industry to the distributor and/or transporter and from these pharmacies and drugstores to the final consumer. The transportation time must also be evaluated year-round. [3, 4, 5, 7, 28, 29]
Our work initiates with a bibliographic review of the main concepts of pharmaceutical attention and assistance, management, pharmaceutical as the manager of the supply chain. After this review, we carried out a statistical study in several companies that encompass partially or totally the logistical chain and the pharmaceutical as the manager.
This study was carried out in the state of São Paulo, encompassing the cities of Campinas, Americana, Jundiaí, Limeira, Valinhos, Vinhedo, Hortolândia and São Paulo. This study analyses the role of pharmaceuticals in the logistic segment, not only as a mere complier to the legislation, but as a manager.
Our data was gathered in transporters, logistic operators and distribution and storage centers. We interviewed a total of 221 companies that work in the pharmaceutical product chain.
Table 1: Data collected company on site and pharmacists
Raised data |
|
Transporters |
145 |
Logistics and Logistics Operator |
12 |
Distributors |
64 |
It has cold chain in operation |
112 |
The It has cold chain in the operation but does not meet |
109 |
Pharmacist gift |
208 |
Pharmaceutical missing |
13 |
It pharmacist substitute or co-responsible |
32 |
Man |
103 |
Womam |
137 |
Age 21 to 30 years |
128 |
Age 31 to 45 years |
84 |
Age above 46 years |
28 |
You do not have post graduation |
112 |
Post graduate in some area |
97 |
Post graduate in logistics |
31 |
Time in function 1 to 4 years |
178 |
Time in function 5 to 9 years |
51 |
Time in the function above 10 years |
11 |
You know what's pharmaceutical management and applies |
28 |
You know what's pharmaceutical management and does not apply |
84 |
Do not know what's pharmaceutical management |
128 |
CRF has regularized |
208 |
SIVISA have regularized |
208 |
AFE has Medicines and regularized inputs |
208 |
AE has Medicines and inputs controlled regularized |
104 |
AFE Cosmetics has regularized |
208 |
AFE has regularized Health Products |
208 |
AFE has regularized Household Cleaning |
56 |
AFE has regularized Food |
38 |
Charter Hall has regularized |
198 |
Has Permit Fire Department regularized |
200 |
General table used for data collection with the pharmacists of respondents.
We used 49 questions involving company type, age of participants, gender, if possessed some kind of post graduation experience of time and company time.

Graphic 1: Percentage of supply chain companies valued between august to september 2015.
Of the 221companies interviewed during the months of August and September 2015, 65.6% are transports, 5.4% logistics and 29% of distributors.
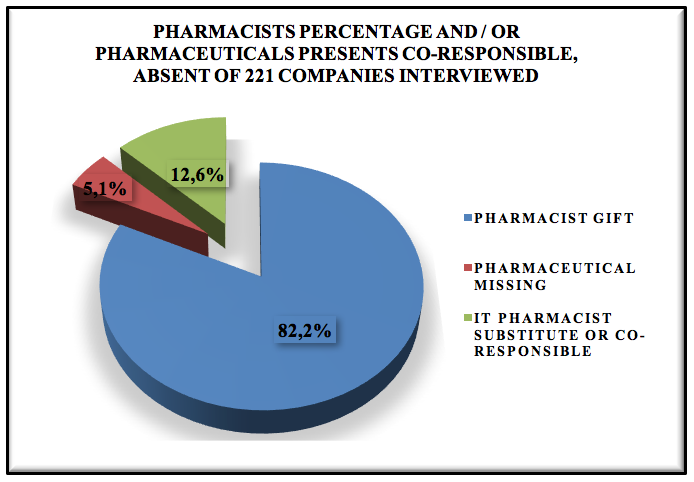
Graphic 2: Pharmacists percentage and / or pharmaceuticals presents co-responsible, absent of 221 companies interviewed.
These companies interviewed 5,1% have no pharmacist present, working out of legal compliance during the interview was realized that there are flaws in the process, where the operating unaware of good practice, do not give importance to this type of process, treat the charge as were any other commodity and that this total lack pharmaceutical carriers are and most are subsidiaries.
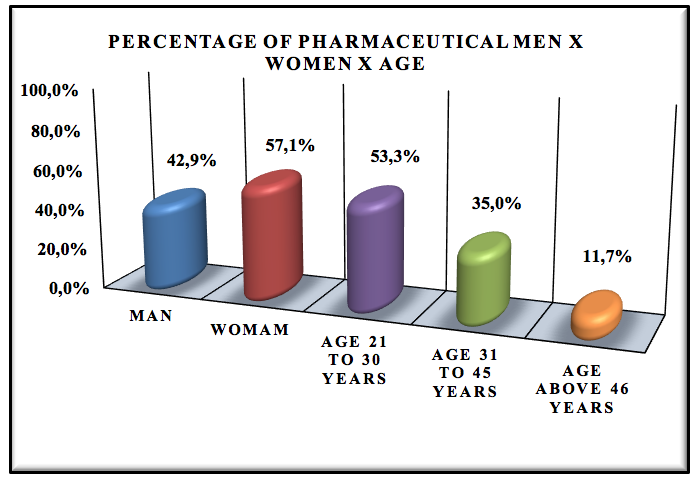
Graphic 3: Percentage of Pharmaceutical Men x Women x Age.
The pharmaceutical profile is 42.9% men to 57.1% women, the prevalence of respondents are between 21-30 years of age, 35.0% has aged 35-41 years old and 11.7% has age above 46 years. We realize that there is predominance of women in the pharmaceutical logistics area because already the course of pharmacy in Brazil have more women than men.
There is the chart that there is also a predominance of newly graduates with little experience in the pharmaceutical logistics market, because they have between 21 to 30 years old.
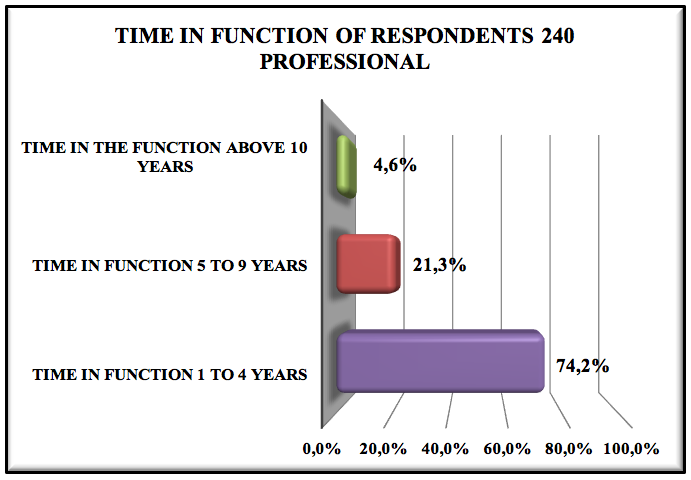
Graphic 4: Time in function of respondents 240 professionals
74.2% of respondents are working in the company 1 to 4 years, 21.3% are working in the company from 5 to 9 years and only 4.6% over ten years, this data meant that we could be experiencing a large turnover pharmaceutical, which can be due to wage policies, benefits, work developed within the company.
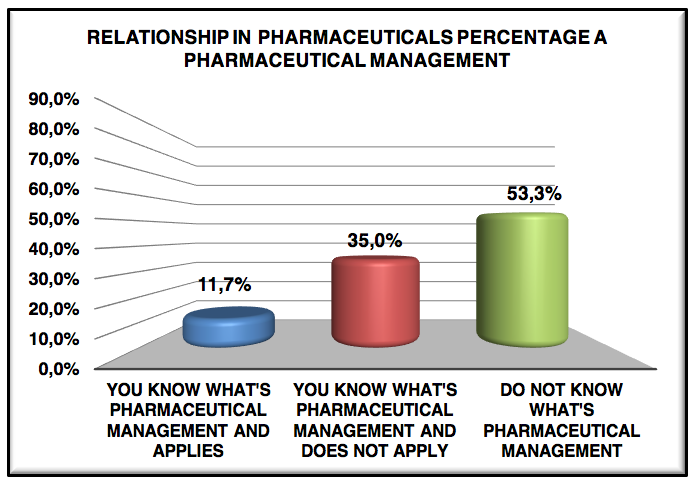
Graphic 5: Relationship in pharmaceuticals percentage a pharmaceutical management
Only 11.7% know what pharmaceutical management and applies, 65.0% know what pharmaceutical management but does not apply and the rest do not have any idea what is management, claim the pharmacists do not know and do not apply to management pharmaceutical is that there is much resistance board, management and operational personnel, out failure during graduation, which is not presented branches of pharmacy, concern still much to drugstores, industrial, hospital pharmacy and clinical analysis.
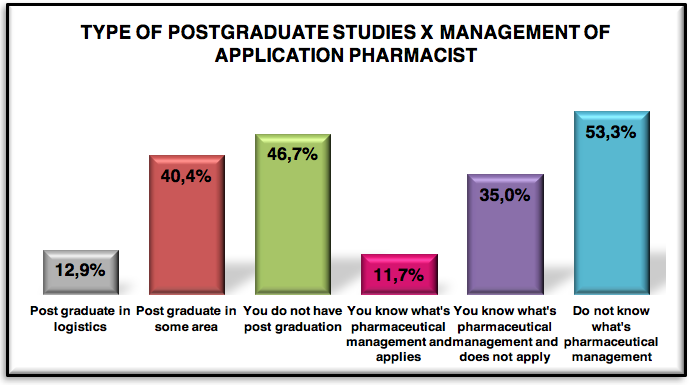
Graphic 6: Graduate type of respondents 221 pharmaceuticals
46.7% made only graduation, so the logistics market, there in great predominance of active pharmaceutical only with graduation, the curriculum of education in Brazil , is not included the matter of pharmaceutical logistics or supply chain.
Of 53.3 %, only 12.9 % have expertise in pharmaceutical logistics and the rest of 40.4 % made a post graduate degree in another area.
Of the 221 interviewed and evaluated companies from August to September, 65.6% were transporters, 5.4% logistics and 29.0% distributors. Only 5.1% of the companies do not have a pharmaceutical, work outside the legal range, have flaws in the process, the staff is unaware of best practices, do not consider important this type of process and treat the load as any other merchandize. Of the companies that do not have a pharmaceutical, 96% are transporters and most of them are branches of bigger companies.
The profile of the pharmaceutical professionals is of 42.7% men to 57.1% women, most of the interviewed are around 21 to 30 years-old. There is also a predominance of recently graduated professionals (46.7%). Most of them have been hired 1-4 years ago (74.2%) and only 46.7% know what pharmaceutical management is. Regarding the graduation of these professionals, 83.7% graduated in private institutions, while 16.3% in public institutions.
Only 46.7% of the interviewed know what is pharmaceutical formation and only 35.4% apply the pharmaceutical management concept in the company. The pharmaceuticals that do not apply the concept in the company reply that they face great resistance from the directory board, managers and operational staff.
On average, the Brazilian pharmaceutical is 32 years old. While in the São Paulo state the average age of the professional is one year older than the national average (33 years old).
The monthly wage of the pharmaceuticals interviewed is:
More than half (62%) of pharmaceuticals interviewed work 20-30 hours per week. While 38% have a work journey of more than 40 hours weekly. Additionally, 47% of all pharmaceuticals are indifferent or unsatisfied with the Brazilian Pharmacy Council (CFF) and the Pharmacy Regional Council of São Paulo (CRF-SP).
Of the 221 companies interviewed, 50.7% have implemented the cold chain in their logistics and follow the norms that guarantee the quality of their product, while 49.3% have the cold chain but do not have the adequate regulations, storages and trucks for transportation and do not monitor moisture and temperature according to the best practices to guarantee the product quality until it reaches the final consumer. The main reply is that the cargo owners do not pay any extra value for this type of service and they guarantee that the period that the merchandise is exposed to these temperatures do not affect or compromise the pharmacokinetics and pharmacodynamics of the molecule.
We can conclude through the data that there’s a wide range of pharmacists recently graduated who has little experience in the logistic area, demonstrate little knowledge of pharmaceutical management and who are just abiding to the legislation in vigor, not worrying too much with the merchandize, with the excuse that the managers or owners don’t want this type of service.
We can also note that in the vast majority of these companies, the directory board along with the managers don’t want to apply the concept due to the cost and the time spent by the pharmacist on the company to develop this type of work.
It’s clear that in the small amount of companies that have 1 to 2 pharmacists who work on average 40 hours per week, the service’s quality is different because besides having post-graduation in the logistic area, they have experience on the job as well as having knowledge of pharmaceutical management and apply this concept in reality bringing several benefits to the companies, such as consumer loyalty, high quality of logistical processes, thus ensuring the quality of the product to the final consumer. The staff is well trained and guided by understanding the process as a whole and not partially, which results in commitment with the merchandize.
In a objective way, it was highlighted that few pharmacists know and apply the concept of pharmaceutical management, linking this function to the pharmaceutical assistance and attentions within hospitals, clinics, pharmacies, dispensaries of Brazilian Unified Health System (SUS in Portuguese) and drugstores, therefore, it’s something that the CFF could begin to work along with the commission of curriculum matrices of both the CFF and the CRF’s so the pharmacists get out of college with a better knowledge of what pharmaceutical management is and its real necessity of application, because we were able to demonstrate that in the few companies that apply this concept of management, the quality of the service is excellent.
Therefore, the pharmaceutical management isn’t only the issue of pharmaceutical assistance or attention applied to the Brazilian market in the present, there are several works in this area involving hospital pharmacies, clinics, pharmacies, among others, ensuring a treatment compliance, highlighting better side effects, fallouts and others.
It’s more than necessary that pharmacists start to know better this concept of pharmaceutical management, not only applied to pharmaceutical assistance and attention, but extend its horizon and apply it to other areas of the pharmaceutical field, such as logistics, transportation, storage, distribution, guaranteeing an effective and efficient product quality up to the final consumer.
BRAZIL. Ministry of Health. Law 5991, from December 17th, 1973. Dispose about the sanitary control of drugs, medications, pharmaceutical supplies and correlated. Brasília, DF. Available on: http://www.anvisa.gov.br/legis/consolidada/lei_5991_73.htm. Accessed in: November 24th, 2014.
BRAZIL. Ministry of Health. Law 6360, from September 23rd, 1976. Dispose about health surveillance which drugs, medications, pharmaceutical supplies and correlated, cosmetics and sanitizers are subjected. Brasília, DF. Available on: http://www.anvisa.gov.br/legis/consolidada/lei_6360_76.pdf. Accessed in: November 24th, 2014.
BRAZIL. Ministry of Health. Secretary of Health Surveillance. Ordinance n.344, from May 12th, 1998. Aproves the technical regulation about substances and medications subjected to special control. Available on: http://bvsms.saude.gov.br/bvs/saudelegis/svs/1998/prt0344_12_05_1998_.html. Accessed in: September 10th, 2014.
BRAZIL. National Council of Pharmacy. Resolution n. 365, from October 2nd, 2001. Disposes about technical pharmaceutical assistance in distributor, representative, importer and exporter of medications, pharmaceutical supplies and correlated. Available on: http://www.crfsp.org.br/legislacao/784-resolucao-365-de-2-de-outubro-de- 2001.html. Accessed in: August 10th, 2014.
BRAZIL. National Council of Pharmacy. Resolution n. 433, from April 26th, 2005. Regulates the role of pharmacists on companies of terrestrial, aerial, railways or fluvial transportation of pharmaceutical products and products for health. Available on: http://www.crfsp.org.br/legislacao/751-resolucao-433-de-26-de-abril-de-2005.html. Accessed on: august 10th, 2014.
BRAZIL. National Health Surveillance Agency (ANVISA in Portuguese). Resolution n. 1, from July 29th, 2005. Aproves the guide to the realization of stability studies. Available on: http://www.anvisa.gov.br/legis/resol/2005/001_05/re.html. Accessed on: April 19th, 2014.
BRAZIL. National Health Surveillance Agency (ANVISA in Portuguese). Resolution n. 204, from November 14th, 2006. Aproves the technical regulation of good distribution and fractionation of pharmaceutical supplies practices. Available on: http://www.anvisa.gov.br/legis/resol/2006/204_06/rdc.html. Accessed on: January 25th, 2014.
BRAZIL. National Health Surveillance Agency (ANVISA in Portuguese). Resolution of the Collegiate Board of Directors n. 17, from April 16th, 2010. Disposes about the Good Practices on Medications’ Making, Brasília, DF, 2014.
CAIXETA-FILHO, José Vicente; MARTINS, Ricardo Silveira (Org.). Gestão Logística do Transporte de Cargas (Logistics Management of Freight Transportation. T.N.: free translation). 1. Ed. 6. reprint. São Paulo: Atlas, 2009.
CONSENSO BRASILEIRO DE ATENÇÃO FARMACÊUTICA - PROPOSTA. Atenção Farmacêutica no Brasil: “Trilhando Caminhos” (BRAZILIAN CONSENSUS OF PHARMACEUTICAL ATTENTION - PROPOSITION. Pharmaceutical attention in Brazil: “Wandering Ways”. T.N.: free translation). Brasília: Pan American Health Organization, 2002. 24p.
DIAS, Marco Aurelio Pereira. Administração de materiais: uma abordagem logística (Material Management: a logistics approach. T.N.: free translation). 4th ed., 12th selection. São Paulo: Atlas, 1993.
DISTRIBUIÇÃO E TRANSPORTES (DISTRIBUTIONS AND TRANSPORTATION). São Paulo: Regional Council of Pharmacy of São Paulo. Comissão Assessora de Distribuição e Transportes (Associated Commission of Distribution and Transportation), ed.2, 2009. Available on: http://www.crfsp.org.br/comissoes-assessoras-/343-um-roteiro-geral-do-ambito- farmaceutico.html. Accessed on: January 20th, 2014.
DORNIER, Philippe-Pierre et al. Logística e operações globais: texto e casos (Logistics and operations: texts and cases). São Paulo: Atlas, 2000.
DUBOC, Marco. Validação de transporte de produtos com temperatura controlada (Validation of product’s transportation with controlled temperature. Revista Controle de Contaminação (Contamination Control Magazine). V.77, p-16-18, 2005.
EUROPEAN COMMISSION. Commission guideline on good distribution pratice of medicinal products for human use, December 31st, 2011. Health and Consumers Directorate-General. Bruxelles, Belgium. 2011.
FIGUEIREDO, Kleber Fossati; FLEURY, Paulo Fernando; WANKE, Peter (Org.).; Marques, Vitor; Lacerda, Leonardo; Ribeiro, Aline. Logística e Gerenciamento da Cadeia de Suprimentos: Planejamento do Fluxo de Produtos e dos Recursos (Logistics and Management of Supply Chain: Flow Planning of Products and Resourses). 1. ed. 3rd reprint. São Paulo: Atlas, 2003.
GRUPO DE INVESTIGACIÓN EN ATENCIÓN FARMACÉUTICA UNIVERSIDAD DE GRANADA. II Consenso de granada (II Granada’s Consensus). Atención-farmacéutica em internet. Available on: http//:www.atencionfarmaceutica.com. Accessed on: December, 13th, 2004.
MENEZES, E.B.B. Atenção farmacêutica em xeque (pharmaceutical attention in check). Rev. Pharm. Bras., v.22, n. p. 28, 2000.
OLIVEIRA, Otávio J. (Org.). Gestão da qualidade: tópicos avançados (Quality Management: advanced topics). São Paulo: Pioneira Thomson Learning, 2004.
OLIVEIRA, A.B.; OYAKAWA, C.N.; MIGUEL, M.D.; ZANIN, S.M.W.; MONTRUCCHIO, D.P. Obstáculos da Atenção Farmacêutica no Brasil (Obstacles of Pharmaceutical Attention in Brazil). Rev. Bras. Ciên. Farm., v. 41, n.4, p.409-413, 2005.
REY, Maria F. Indicadores de desempenho logístico (Indicator of logistical performance). Revista Logmam (Logmam Magazine), May/June, 1999.
SANTICH, I.R. Enfoque integral del proceso de suministro de medicamentos y otros insumos críticos para el sector salud. Washington, D.C.: OPAS, 1989.
SANTORO, Maria Inês Rocha Maritello. Introdução ao Controle de Qualidade de Medicamentos (Introduction to quality control of medication). São Paulo: Atheneu Editora (Atheneu Editor) - Editora da Universidade de São Paulo Editor of University of São Paulo), 1988.
SINK, D. Scott; TURTTLE, Thomas C. Planejamento e medição para a performance (Planning and measurement for performance). Rio de Janeiro: Ed. Qualitymark, 1993.
VECINA NETO, Gonzalo. Gestão de recursos materiais e de medicamentos (Management of material resources and medication). São Paulo: USP/School of Public Health, 1998. v. 12. (Health & Citizenship Series).
WEXMAN, S. El proceso de adquisición de medicamentos en el sector público. Washington, D.C.: OPAS/OMS, 1989.
WORLD HEALTH ORGANIZATION. Model guidance for the storage and transport of time-and-temperature-sensive pharmaceutical products. Technical Report Series, n. 961, annex 9, p.324-367. Geneva, Switzerland, 2011.
ZARDO, Humberto. Boas práticas de armazenamento, transporte e distribuição de medicamentos: contribuição para visão integrada das necessidades (Good practices of storage, transport and distribution of medication: contribution to the integrated vision of necessities). Instituto Racine (Racine Institute), 2012. In: Revista fármacos e medicamentos ( Medicine and Medication Magazine), v. 66, August/September/October, 2011. Available in: http://www.racine.com.br/index.php. Accessed on February 10th, 2014.
1. (Aeroporto de Viracopos, Campinas, SP, Brasil) Email: logisticafarmaceutica@hotmail.com
2. (School of Applied Sciences, State University of Campinas, UNICAMP, Limeira, SP) Email: ieda.makiya@fca.unicamp.br
3. (School of Applied Sciences, State University of Campinas, UNICAMP, Limeira, SP) Email: giocondo.cesar@gmail.com