

Vol. 38 (Nº 48) Year 2017. Page 32
Dmitry PODOPRIGORA 1; Liliya SAYCHENKO 2
Received: 30/09/2017 • Approved: 05/10/2017
3. Data, Analysis, and Results
ABSTRACT: This article is dedicated to the development of acid composition for bottom-hole formation zone treatment of low-permeability polymictic sandstone reservoir at high temperatures (95 oC). The use of traditional formulation of mud acid for these formations was ineffective. Insoluble precipitates may sediment at the reaction of fluorine-containing agents with the carbonate mineral constituents. The high response rate of the traditional formulation of mud acid with minerals does not allow deep processing of productive horizon. A known method for reducing the response rate of acid composition with rock-forming minerals and minimize precipitation is a partial or complete replacement of hydrochloric acid (HCl) on the organic acids. These acids (acetic or formic) have a lower concentration of hydrogen ions in comparison with the HCl and low response rate. In the process of conducting laboratory experiments on the development of the acidic composition were used standard techniques to determine the extent and rate of dissolution of quartz and marble adopted in the oil and gas industry, as well as determining the ability to retain precipitation of insoluble calcium fluoride. Using the developed composition allows significantly increase the depth of processing. The experiment to determine the ability to retain precipitation of insoluble calcium fluoride showed that the composition doesn’t sediment. Thus, the developed acid composition showed a high efficiency based on laboratory tests. |
RESUMEN: Este artículo está dedicado al desarrollo de la composición ácida para el tratamiento de la zona de formación de fondo-agujero del depósito de arenisca polimictica de baja permeabilidad a altas temperaturas (95 oC). El uso de la formulación tradicional de ácido de barro para estas formaciones fue ineficaz. Los precipitados insolubles pueden sedimentar a la reacción de agentes que contienen flúor con los constituyentes minerales de carbonato. La alta tasa de respuesta de la formulación tradicional de ácido de barro con minerales no permite el procesamiento profundo del horizonte productivo. Un método conocido para reducir la tasa de respuesta de la composición ácida con minerales formadores de rocas y minimizar la precipitación es una sustitución parcial o completa del ácido clorhídrico (HCl) sobre los ácidos orgánicos. Estos ácidos (acético o fórmico) tienen una concentración más baja de iones hidrógeno en comparación con el HCl y baja tasa de respuesta. En el proceso de realización de experimentos de laboratorio sobre el desarrollo de la composición ácida se utilizaron técnicas estándar para determinar la extensión y tasa de disolución de cuarzo y mármol adoptada en la industria del petróleo y el gas, así como determinar la capacidad de retener la precipitación de calcio insoluble fluoruro. El uso de la composición desarrollada permite aumentar significativamente la profundidad de procesamiento. El experimento para determinar la capacidad de retener la precipitación de fluoruro de calcio insoluble demostró que la composición no sedimenta. Así, la composición ácida desarrollada mostró una alta eficiencia basada en ensayos de laboratorio. |
Well completion and development of oil and gas wells processes are extremely important, because after these stages their operation begins. However, existing methods of development wells do not solve the problem, resulting in the process of drilling and completion, for example, the bottomhole formation zone colmatation. Laboratory filtration studies of the bottomhole formation zone colmatation problem are considered in the work (Podoprigora, 2015). There are such methods as hydraulic fracturing or acid treatment used for the development of the wells after drilling for this reason in recent years. In addition to the issues of man-made plan, the well efficiency is greatly influenced by the geological structure of the productive horizon (Glushchenko and Ptashko, 2014; Khizhnyak et al., 2015, Rogachev et al., 2007).
Usually acid composition containing hydrofluoric acid uses for acid treatment of low-permeability sandstone formations. Most often a mixture of hydrochloric acid (10-12%) and hydrofluoric acid (3-6%) are used at treatment of such reservoirs, but the success of mud acid treatments do not exceed 50% (Magadova and Silin, 2003).
As shows the analysis of the resource base of the Russian Federation a large part of unconventional oil (about 60 %) are concentrated in low-permeability reservoirs, and the productive deposits of the Tyumen Suite. Productive sediments of these objects are presented by polymictic sandstones with clay-carbonate cement to 25-30 % (Podoprigora, 2016). These objects are just starting to enter in active development, respectively, for their development it is necessary to attract new technologies
In this study, acid composition specifically developed for treatment polymictic carbonated sandstones. The use of traditional formulation of mud acid for these formations was ineffective. Insoluble precipitates may sediment at the reaction of fluorine-containing agents with the carbonate mineral constituents (under certain conditions) (Magadova et al., 2010). In this paper we investigate another important aspect - the possibility of regulating the response rate of the new composition with the basic rock-forming minerals of the formation. The high response rate of the traditional formulation of mud acid with minerals does not allow deep processing of productive horizon (Magadova and Silin, 2010).
A known method for reducing the response rate of acid composition with rock-forming minerals and minimize precipitation is a partial or complete replacement of hydrochloric acid (HCl) on the organic acids. These acids (acetic or formic) have a lower concentration of hydrogen ions in comparison with the HCl and low response rate (Abrams et al., 1983; Galkin et al., 2014).
So employees of national company "Khimeko-GANG" (Moscow) was developed acid-cut clay mud composition "Himeko TC-2" water-glycerin basis of prolonged action that includes a surfactant and hydrofluoboric acid (Glushchenko and Ptashko, 2014; Magadov et al., 2006). After dilution of the acid in 6 times with fresh water, it has low interfacial tension on the border with kerosene and slow speed of reaction with the aluminosilicates.
At the same time work (Glushchenko and Silin, 2010) refers to identical accumulation of precipitation of silicates of alkaline earth metals by the reaction of acidic compounds with clay minerals. Authors conclude that according to foreign sources application of hydrofluoboric acid in terrigenous reservoirs treatment is not necessary.
Work (Hall, 1978) shows the comparison of the change in the filtration properties of artificial sandstone, which includes 95 % sand and 5 % smectite, after filtration through them the two acid solutions (12 % HCl + 3 % HF and 7.5% HCl + 2,8 % NH4F). Due to minimize the processes associated with secondary sedimentation acid solution containing ammonium fluoride (NH4F) increased the permeability of the core at 90 %.
According to research by the solubility of terrigenous rocks with high carbonate content of several fields in Western Siberia Sidorovsky V.A. recommended for use the following acid composition: 10 % HCl + 3 % NH4HF2 (Sidorov, 1971).
Found that a mixture of sulfamic acid with ammonium bifluoride reacts with terrigenous formation much slower than solutions containing hydrochloric acid and ammonium bifluoride at a temperature of 80 °C (Glazkov & Marichev, 1980).
According to (Gavrilenko et al., 2007) H2SiF6 and its mixture with hydrochloric acid during the reaction with the aluminosilicates yield the difficulty soluble precipitation, with increasing temperature the processes of sedimentation increase.
In work (Sparlin, 1982) the interaction of phosphate-based composition H3PO4 "turflo" in combination with HF or ammonium salts of hydrofluoric acid by the reaction with the aluminosilicates and carbonates are investigated. As a result of these experiments it was established the distinctive feature of this composition, expressed in a sharp decrease in the rate of reaction with carbonates and unchanging character of the reaction of hydrofluoric acid in sandstone rock. The authors of (Glushchenko and Silin, 2010) explain the effect of a mixture of phosphoric and hydrofluoric acid on carbonates by the coating of the surface calcium fluoride and phosphate with subsequent consumption of acids mainly in the hydrolysis, not the dissolution of carbonates.
To increase the permeability of reservoir rocks in the bottomhole formation zone at fields with high reservoir temperatures foreign experts (Ziauddin, 2005) proposed the technology of injection of 10 % solution of acetic acid (CH3COOH), and then aqueous solution containing 6 % HBO3 + 3 % citric acid + 1 % HF. This acidic solution is characterized by a uniform rate of generation of secondary precipitation during movement into the formation due to the low values of pH, supported by the acetic acid on the displacement front.
Known composition, which can be used when high formation temperatures, contains a 10% aqueous solution of CH3COOH and 0,1-0,2% of HF or ammonium salts of HF, which virtually eliminates the possibility of secondary sedimentation (Wehunt, 1994).
According to foreign scientists (Al-kharti et al., 2008; Tuedor et al., 2006) effective way stimulation in wells with high temperatures is the use of chelating agents, which give the opportunity to intensify the inflow conditions, when the use of mineral acids is not valid. The chelating agents are used to prevent sedimentation of the metal compounds, and they can slowly affect rock-forming minerals and have a low rate of corrosion.
Thus only the first steps were made in the development of acid compositions for high-temperature low-permeability terrigenous reservoirs. Therefore the development of a new acid composition for these conditions is an urgent task not only for domestic industry but also for the world.
The experiment method of determining the ability of acid composition to retain precipitation of insoluble calcium fluoride were developed on the basis of the materials presented in (Podoprigora, 2017; Silin et al., 2011).
In the process of conducting the experiment, we used disks (d=3 cm h=1 cm), made of marble. Next, the discs were washed with distilled water, dried to constant mass and cooled for 2 hours in a desiccator. Then three banks warmed to the temperature of the experiment, the subjects acid composition exceeding the surface area of the disk in 2.5 times was placed one marble disk. The time of the experiment was 1, 2 and 3 hours.
Each disk after the completion of the experiment, washed with an aqueous solution of sodium hydroxide and water, dried to constant mass and cooled in a desiccator and then weighed to determine weight loss of the marble disk.
The experiment method procedure of determining response rate with the quartz and marble and the amount of dissolved minerals consisted of the following/
During the test, we used a quartz or marble plates. Before the experiment, the plates are washed with water and wiped with acetone, and then dried to constant weight and weighed on a laboratory scales. Later in the six jars with lids the quartz plate are hunged, however, they do not touch the bottom and walls of the jar. Then in each jar was filled, the subject acid composition, heated to the temperature of the experiment, exceeding the surface area of the quartz plate in 2.5 times.
The time of the experiment was 15, 30, 60, 120 and 180 minutes.
Each plate after completion of the experiment, washed with an aqueous solution of sodium hydroxide and water, dried to constant mass and cooled in a desiccator and then weighed.
At the first stage of research five compositions were tested: standard mud acid (10% HCl + 1,5% HF) – composition 1, mixtures with acidity equivalent of 3, 6, 8, 10% hydrochloric acid (in which 50% HCl was replaced by formic acid ) supplemented with 1% of the ammonium bifluoride - compositions 2, 3, 4 and 5, respectively. The response rate with the quartz and the amount of dissolved minerals were determined for these compositions (Fig. 1, 2). These experiments showed that the amount of organic acid in the composition affects essentially on the rate of dissolution during the test, i.e. more organic formic acid is contain in the composition, the more uniform is the dissolution of the mineral.
According to the number of dissolved silica in 3 hours the following results were obtained: composition 1 - 9.7% of silica; composition 2 - 9.4%; composition 3 - 9.4%; composition 4 - 9.4%; composition 5 - 9.9%. Thus, concentration of the organic acid does not affect the amount of dissolved silica.
Then, compositions 1, 2, 3, 4 and 5 were tested to determine the reactivity related to carbonates. For this marble discs were subjected to an acid treatment, and the response rate and the amount of dissolved carbonate were determined (Fig. 3, 4).
The results showed that in relation to carbonate, the response rate and the amount of dissolved minerals are greatly influenced by increasing the concentration of hydrochloric and organic acids. The greater the concentration of acids, the faster the response rate of acid composition with marbles in the initial moment of the reaction, and then it sharply decelerates during the experiment. The amount of dissolved marble during the 3 hours was: composition 1 - 24.5%; composition 2 - 12%; composition 3 - 21%; composition 4 - 26%; composition 5 - 30.5%. In addition, deacidification of the composition 1 (10% HCl), in the reaction with marble, occurs within 30 minutes of the experiment, and compositions which include formic acid react with the mineral throughout the experiment. But in the case of using organic acid composition is highly depleted in the first 30 minutes of the reaction and the rate of dissolution is significantly reduced.
To resolve all identified problems, a new acidic composition 6 was developed in the laboratory Enhanced oil recovery of Mining University (St-Petersburg). Composition 6 contains hydrochloric, formic acid, ammonium bifluoride, mixture of surfactants, iron deflocculant, corrosion inhibitor and versene.
Figures 1, 2, 3, 4 present the results of studies on the solubility and response rate of quartz and marble in acid compositions 1,2,3,4,5 as well as a new acid composition 6 (curve 6\ it is only basis of the developed acid composition (without surfactants and corrosion inhibitor).
Figure 1. The dissolution rate of quartz at 95 °C
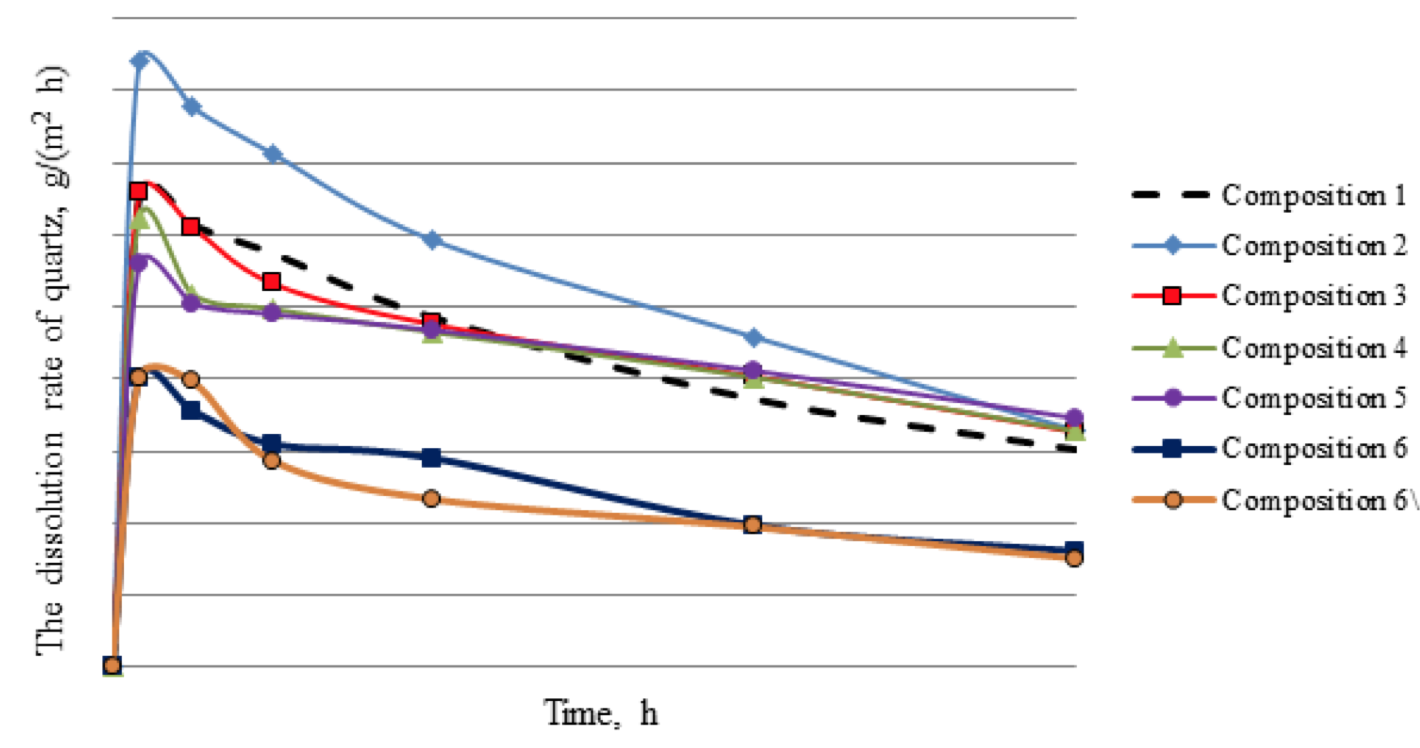
Figure 2. The amount of dissolved quartz at 95 ° C
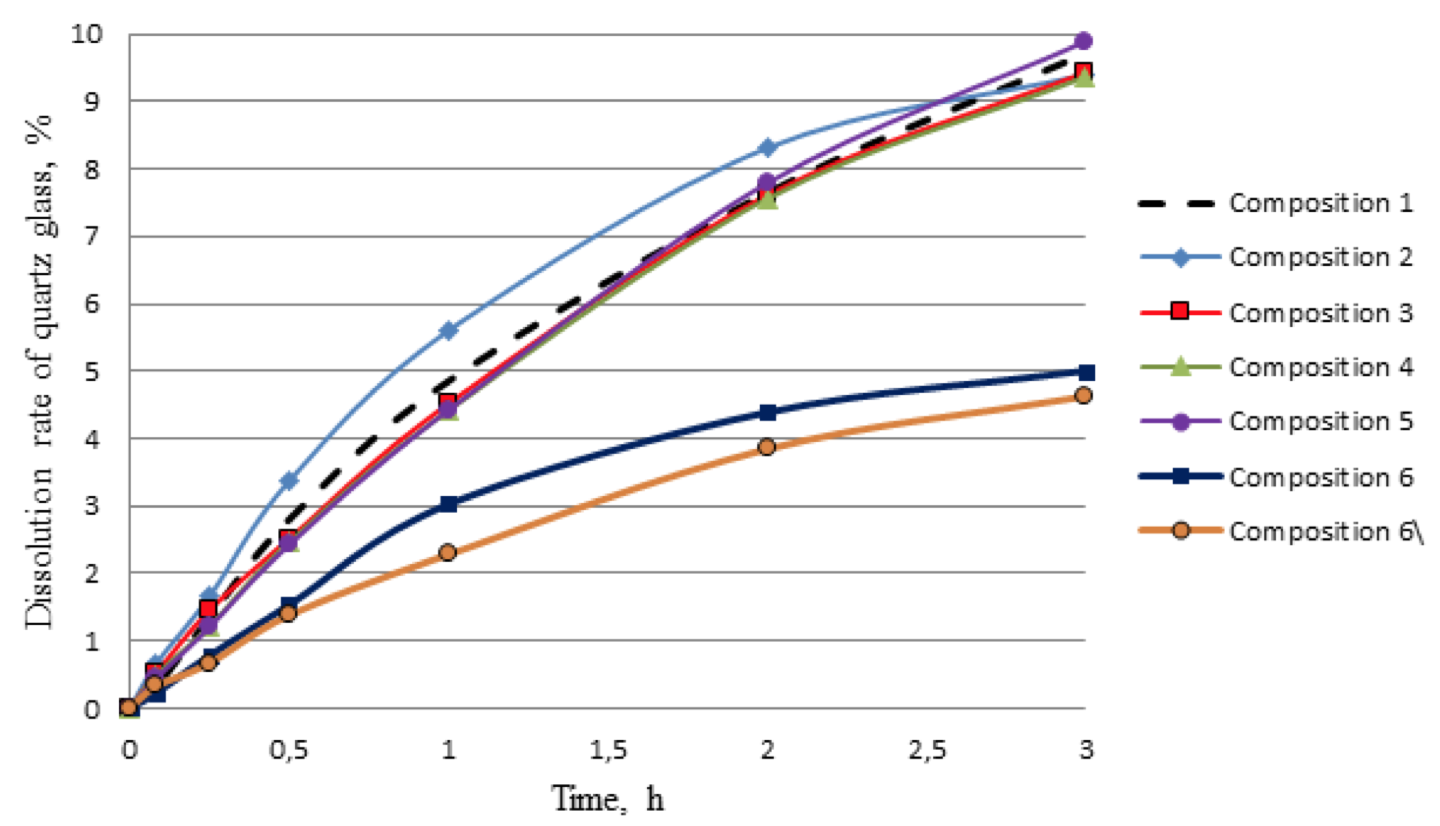
Analyzing the dependence it should be noted that the developed acid composition (lines 6, 6 \) reacts with quartz slower, compared with commonly used acids in the field (compositions 1-5). The total amount of dissolved mineral by developed composition is halved; it is 5% for 3 hours. This at first glance may seem negative, but conducted experiment to determine the ability to retain precipitation of insoluble calcium fluoride (result from contact of fluoride contained acids with carbonates according to reaction 1, 2) has shown that the compositions 6 and 6 \ practically do not give such insoluble precipitations, unlike acids 1, 2, 3, 4 and 5.
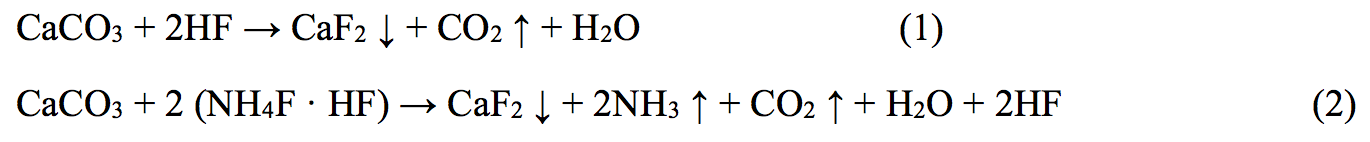
Figure 3. The rate of dissolution of marble at 95 ° C
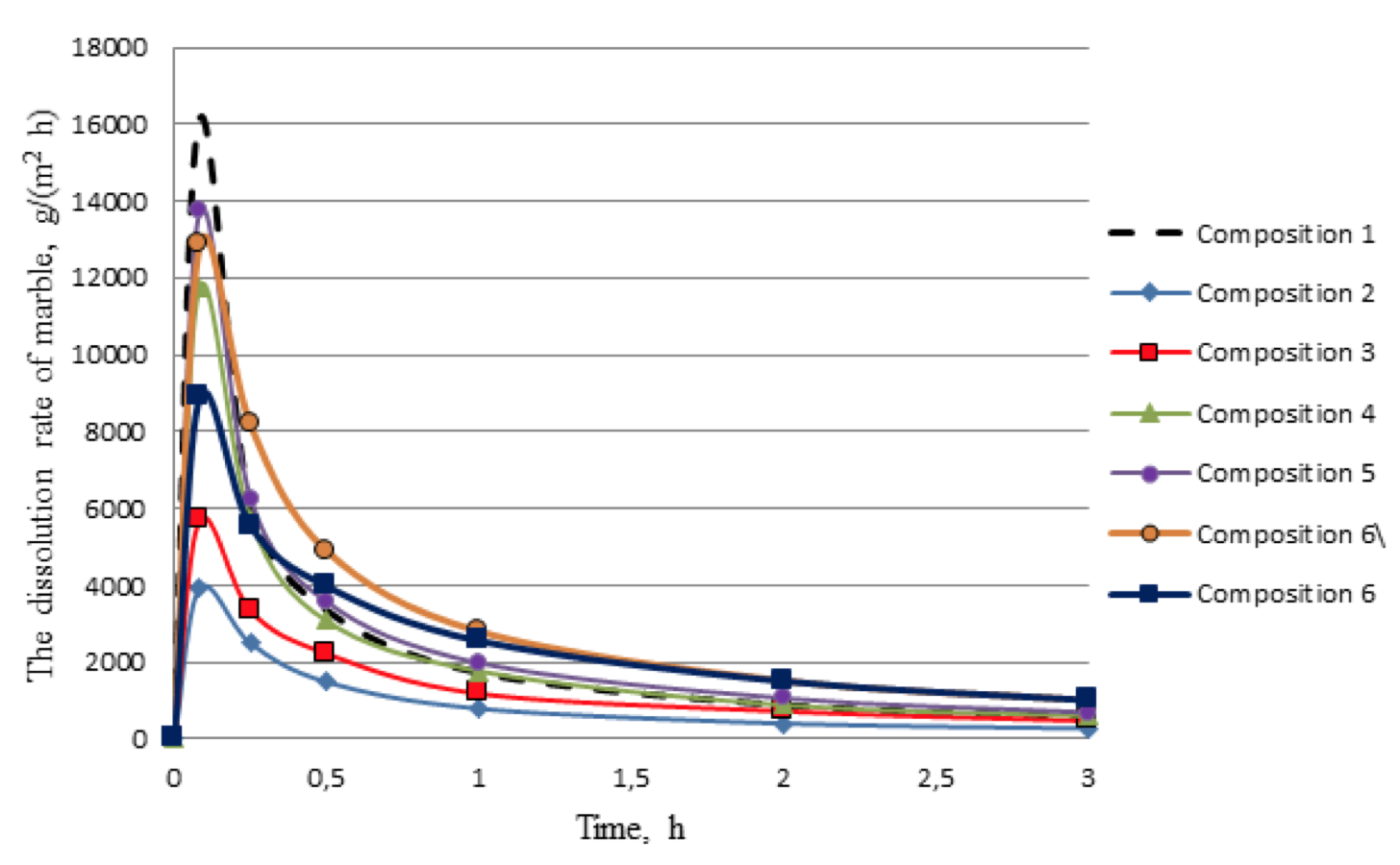
Figure 3 shows that the composition 6 achieves reduction of the response rate of the acid composition with the carbonate component of the formation at the initial time of the reaction. Another positive feature of the composition 6 is that for 30, 60, 120 and 180 minutes from the reaction with marble response rate does not decrease so much as in acid compositions 1-5. Therefore it can be assumed that the treatment of carbonized polymictic sandstone by composition 6 will allow significantly increase the depth of processing. The graph of the amount of dissolved marble to time for all tested compositions in fig. 4 is an additional argument in this regard.
Figure 4. The amount of dissolved marble at 95 ° C
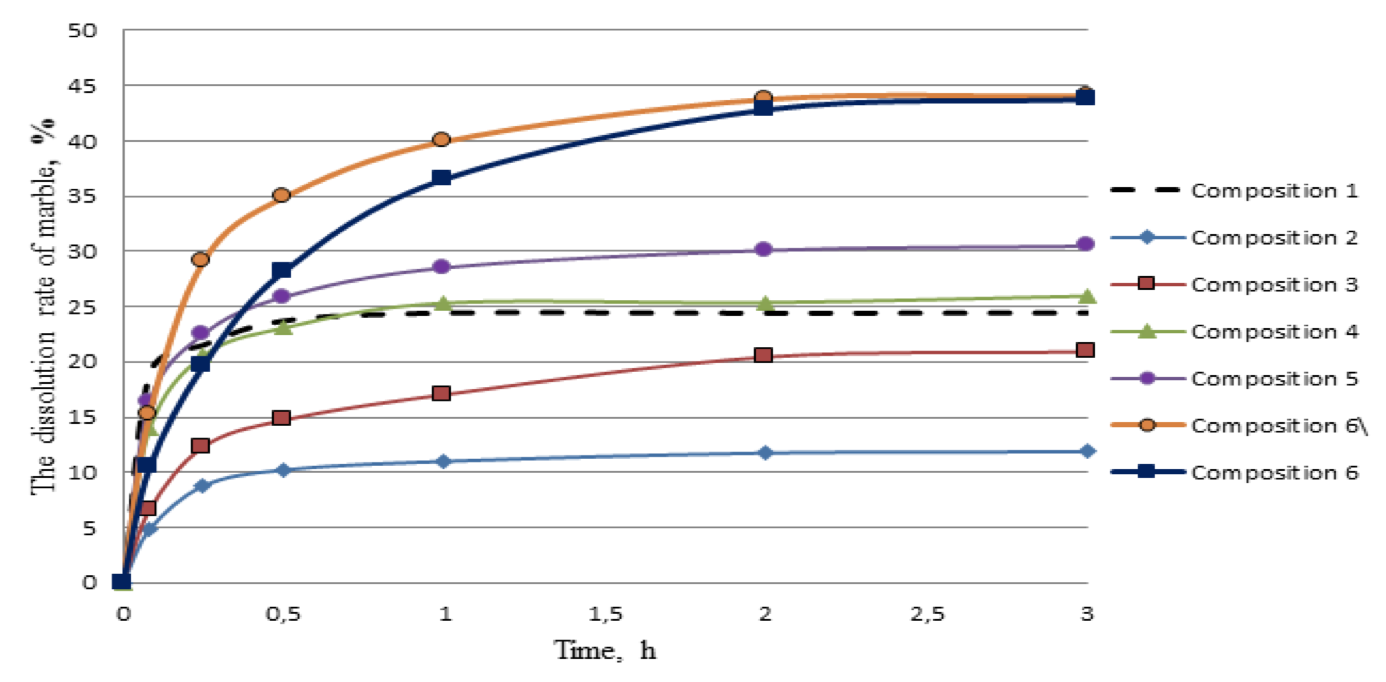
Figure 4 shows that the composition 6 the most uniformly dissolves the carbonate component of the formation within 2 hours of the experiment. At the third hour of the experiment developed composition dissolves a small amount of marble, like the rest of the compositions.
Test to determine the ability to retain precipitation of insoluble calcium fluoride on time showed that compositions 1, 2, 3, 4, 5 and 6, much inferior to the compositions 6 and 6 \, which practically do not give undesirable precipitation during the reaction with the carbonate component. So the ability to retain precipitation of compositions 6\ and 6 was 97 and 99%, respectively, while it is not exceed 85% for the remaining acid compositions.
Filtration tests were conducted using an unsteady state relative permeameter AutoFlood 700 (Vinci technologies) in conditions as close to the reservoir.
For these experiments, a composite model of the three cores, representing polymictic sandstone with clay-carbonate cement, selected on one of the fields in Western Siberia. At the same time initial permeability to the kerosene was determine, then in the opposite direction at a constant repression of 20 atm. pumped 3% aqueous solution of KCl, which is used as the basis of many drilling muds, to simulate penetration process of the mud filtrate in the primary and secondary drilling in, after which the permeability to kerosene of the composite model and damage factor were newly determined. At the final stage, developed acid composition was pumped in an amount of five pore volumes and the final permeability of the composite model to kerosene was determined (kerosene filtration was performed to stabilize the pressure gradient).
The conditions of the experiment: temperature - 95 ° C, confining pressure - 14 MPa, izoviskozic model of the oil - kerosene.
Parameters of the composite model: the length of the composite model - 9.0 cm; the diameter of the composite model - 3.0 cm; pore volume - 11.3 cm3; the initial permeability of the composite model to kerosene at 95 °C - 3.1∙10-15 м2.
The results of the filtration studies are presented in Figure 5.
Figure 5. Dependency of the injection pressure gradients of kerosene,
mud filtrate and developed acid composition on the amount pumped pore volumes
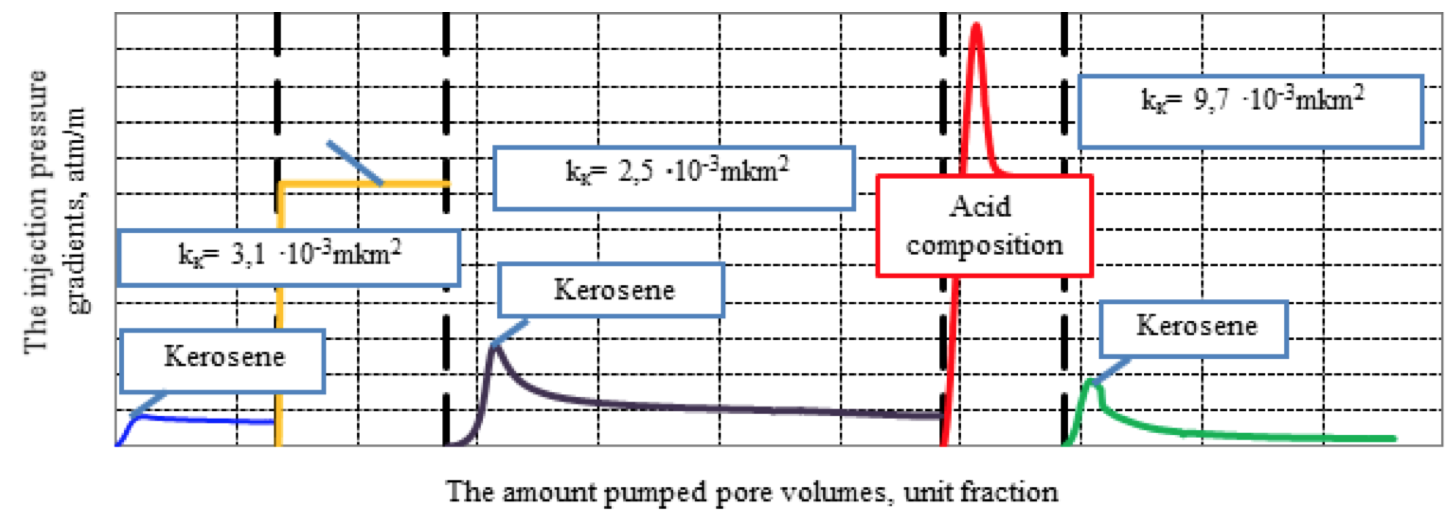
Filtration experiment showed that an aqueous solution of 3% KCl composite model deteriorates the permeability by 19%. Injection of the developed acid composition not only restores the permeability, but also improves it by 3.1 times compared with the initial (Podoprigora, 2015a; 2015b).
A similar problem was solved recently in work (Tsygankov, 2011), which describes the development of acidic composition for use in conditions of terrigenous reservoirs with high carbonate content. When comparing the results of the filtration experiments, it was found that the composition proposed in work (Tsygankov, 2011) improves the permeability on 7.8 % , at the time, as developed acid composition increases the permeability of 3.1 times, indicating that the advantage of using the latter.
Developed acid composition according to the results of laboratory tests showed improved physical and chemical parameters of the dissolution of the main components of minerals polymictic carbonated sandstone (quartz and carbonate dissolution rate slowdown, uniform dissolution, ability to retain precipitation). The likelihood of colmatage precipitation is significantly reduced. Conducted experiments on modeling of acid treatment of the polymictic sandstone after mud filtrate damage have shown that developed acid composition not only restores the filtration properties, but also improves the permeability by 3.1 times compared with the initial. On the basis of obtained results we can recommend developed acid composition for the pilot test of the acid treatment in the development of oil wells in the complicated high-low permeability reservoirs.
Abrams, A., R. F. Scheuerman and C. C. Templeton, 1983. Higher-pH Acid Stimulation System. Journal of Petroleum Technology, 35: 2175-2184.
Al-kharti, S, O. A. Bastos and M. Samuel, 2008.The possibility of intensification of the flow in high temperature wells. Oil and gas review, 4: 66-79.
Galkin, V.I., G.P. Khizhnyak, A.M. Amirov and E.A. Gladkikh, 2014. Evaluation of the impact of acidic compounds in the core using regression analysis. Vestnik PNIPU. Geology. Oil and gas business, 13: 38-48.
Gavrilenko, A.I., N. Demyanenko and V. G. Pusenkov, 2007. New compositions for the treatment of terrigenous reservoirs of the fields of the Republic of Belarus. The Search for and development of petroleum resources of the Republic of Belarus, 6: 192-201.
Glazkov, A.A. and F. N. Marichev, 1980. On the possible use of sulfamic acid for the treatment of terrigenous reservoirs. Oilfield business, 7: 35-37.
Glushchenko, V.N. and O. A. Ptashko, 2014. Filtration studies of new compounds for the treatment of acid carbonate reservoirs. Vestnik PNIPU. Geology. Oil and gas business, 11: 46-56.
Glushchenko, V.N. and M. A. Silin, 2010. Oilfield chemistry. Acid treatment of well, Moskow: Interkontakt Nauka, 4: 703.
Hall, B. E., 1978. A new technique for generating in-situ hydrofluoric acid for deep clay damage removal. Journal of Petroleum Technology, 30(09): 1-220.
Khizhnyak, G.P., A. M. Amir and E. A. Gladkikh, 2015. Laboratory tests of composition DEEPA. Vestnik PNIPU. Geology. Oil and gas business, 14: 18-31.
Magadova, L.A. and M. A. Silin, 2003. Acid composition "Himeko TK-2" for the low permeability terrigenous reservoirs. Oil Industry, 5: 80-81.
Magadov, R.S., L. A. Magadova and V. M. Zaitsev, 2006. Acid composition Himeko TK-2 to increase productivity of wells in low-permeability terrigenous reservoirs of the Uzen field. Oilfield business, 9: 21-25.
Magadova, L.A., M. A. Silin, V. A. Tsygankov and M. M. Mukhin, 2010. Acidic composition for increasing the productivity of wells with low permeability clastic reservoirs with a high content of carbonate. Technology of oil and gas, 1: 41-45.
Podoprigora, D.G., 2015a. Development acidizing composition to conditions of high productive layers with polymictic pedocalcic sandstones. Actual problems of science and technology: proceedings of the VIII International Scientific and Practical Conference. Young Scientists: 3 tons Ufa UGNTU Publisher: 57-59.
Podoprigora, D.G., 2015b. Laboratory studies of acid compounds for conditions of high productive layers with pedocalcic sandstones. Abstracts of the I International School-conference of students, graduate students and young scientists "Biomedical materials and technologies of the XXI century". Kazan: Publishing House of Kazan. University Press: 274.
Podoprigora, D.G., A. V. Petukhov and O. V. Syuzev, 2015. Laboratory studies of changes in filtration-capacitive properties of polymictic sandstones while drilling in polymer mud. Neftegazovaya Geologiya. Theory and practice, 1 (access: http://ngtp.ru/rub/12/10_2015.pdf)
Podoprigora, D. G., 2016. Substantiation of technology of acid development of high-temperature low-permeability terrigenous reservoirs with high carbonate content: dissertation. Podoprigora Dmitry Georgievich, Saint Petersburg: 123.
Podoprigora, D., 2017. Selection of the acidizing compositions for use in terrigenous reservoirs with high carbonate content. International Journal of Applied Engineering Research, 12(2): 249 – 255.
Rogachev M., Mardashov D. and Strizhnev K. 2007. The development of technologies of well killing and stimulation of oil wells underground repair. Proceedings of the Mining Institute, 173: 20 – 22.
Sidorov, V.A., 1971. Pinacolone treatment of wells in Western Siberia. Oilfield business, 11: 32-35.
Silin, M.A., L. A. Magadova and V. A. Tsygankov, 2011. Acidizing and testing techniques acid compositions: a textbook for university students. M.: Gubkin Russian State University of Oil and Gas: 120.
Sparlin, D., 1982. New acid system for processing limestone Sandstone. Oil, gas and petrochemistry abroad, 12: 17-19.
Tsygankov, V.A., 2011. Development of acidizing compositions for low-permeability terrigenous reservoirs with high content of carbonates: dissertation ... of candidate of technical Sciences. Moscow: 162.
Tuedor, F. E. et al., 2006. A breakthrough fluid technology in stimulation of sandstone reservoirs. SPE International Symposium and Exhibition on Formation Damage Control. Society of Petroleum Engineers.
Wehunt, C. D., 1994. Method to increase the flow capacity of a geologic formation : пат. 5375660.
Ziauddin, М., 2005. Method for characterizing secondary and tertiary reactions using reservoir cores. SPEPF, 20(2): 106-114.
1. Saint-Petersburg mining university, Russia, Saint-Petersburg, 21-st line, 2. Email: podoprigorad@yahoo.com
2. Saint-Petersburg mining university, Russia, Saint-Petersburg, 21-st line, 2