

Vol. 38 (Nº 53) Ano 2017. Pág. 4
Cristine Bastos do AMARANTE 1; Antonio Rodrigues FERNANDES 2; Josemar Moreira VASCONCELOS 3; Nilvan Carvalho MELO 4; Pedro Daniel de OLIVEIRA 5; Alba Lúcia Ferreira de Almeida LINS 6; Raimundo Junior da Rocha BATISTA 7; Lourival da Silva RIBEIRO JUNIOR 8; Fernando Antonio de Castro GONÇALVES 9; Wander Gustavo BOTERO 10; Everton Leandro Santos AMARAL 11; Anderson de Santana BOTELHO 12
Recebido: 26/06/2017 • Aprovado: 18/07/2017
ABSTRACT: Concentrations of Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ in sediments, water and different tissues of the Montrichardia linifera growing naturally in Guama river (Belém, "Metropolis of the Amazon", Brazil) were investigated. The aim was to define which plant tissues exhibit the greatest accumulation and find possibles differences on the minerals composition among the collection site (riverbank and igapo). M. linifera accumulated more nutrients on the riverbank than igapo (exception for Zn2+). The plant showed almost two times more Mn2+ (in the leaves) and four times more Fe3+ (in the roots) on the riverbank that on the igapo, which in turn accumulates five times more Zn2+ (roots and leaves) than on the riverbank. So, there is a potential interest for use this specie in phytoremediation studies of polluted waters. |
RESUMO: Foram investigadas concentrações de Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ e Mn2+ em sedimentos, água e diferentes tecidos da Montrichardia linifera que crescem naturalmente no rio Guamá (Belém, "Metrópole da Amazônia", Brasil). O objetivo foi definir quais os tecidos das plantas que apresentam a maior acumulação e encontrar possíveis diferenças na composição dos minerais no local de coleta (margem do rio e igapó). A M. linifera acumulou mais nutrientes na margem do rio do que no igapó (exceção para Zn2+). A planta mostrou quase duas vezes mais Mn2+ (nas folhas) e quatro vezes mais Fe3 + (nas raízes) na margem do rio que no igapó, que por sua vez acumula cinco vezes mais Zn2+ (raízes e folhas) do que na margem do rio. Portanto, há um interesse potencial para usar essa espécie em estudos de correção de águas poluídas. |
Water pollution is nominally one of the most dangerous hazards on both developing and developed countries (FAWZY, BADR, EL-KHATIB & ABO-EL-KASSEM, 2012). In a series of studies on the environmental impacts inflicted upon the Amazonian region by anthropogenic activities, environmental contamination by metals is considered one of the worst problems affecting the Amazonian ecosystem (SERUDO, OLIVEIRA, ROCHA, PATERLINI, ROSA, SILVA & BOTERO, 2007).
In the Amazon, Guama River is also an example of environmental problem. This river supplies the city of Belem (capital of Para State, North Region of Brazil) with 1.425.923 inhabitants (RIBEIRO, 2004). The city is the entrance gate to Amazon River and known as the "Metropolis of the Amazon", it is headquarters of the Metropolitan Region of Belem that with a population of 2.437.297 inhabitants (IBGE), is the 2nd most populous of the region, 13th of Brazil and 177th of world, representing the largest urban agglomeration in the region (MERCÊS & BASTOS, 2011). Guama river supplies about 75% of all water consumed in Belem, but 11 of its tributaries are contaminated by irregular disposal of urban waste. In the north region of the Brazil, about 8.8 million people don’t have sewer collector network, of which 60% this concentrated in Para State (SANTOS, HOLANDA, PEREIRA, RODRIGUES, PEREIRA & MESQUITA, 2014).
It has been known for long time that aquatic plants, both living and dead, are heavy metal accumulators and, therefore, the use of aquatic plants for the removal of heavy metals from wastewater has gained high interest (FAWZY, BADR, EL-KHATIB & ABO-EL-KASSEM, 2012; KUYUCAK & VOLESKY, 1988). Moreover, treatment systems with aquatic plants are low-cost technologies and can be adopted by developing countries for recycling/treatment of waste water, especially contaminated by heavy/toxic metals (FAWZY, BADR, EL-KHATIB &ABO-EL-KASSEM, 2012; FIGUEIRA & RIBEIRO, 2005). This technology is considered as an alternative solution for conventional methods to clean up heavy metals from contaminated waters (FAWZY, BADR, EL-KHATIB &ABO-EL-KASSEM, 2012; KHAMBHATY, MODY, BASHA & JHA, 2009), applied at various industrial events (VERMA, GUPTA & RAI, 2005). This technology can be used to treat both organic and inorganic pollutants present in soil (solid substrate), water (liquid substrate), or on the air (SALT, SMITH & RASKIN, 1998).
A recent study at Nile River showed that the aquatic macrophytes Ceratophyllum demersum, Eichhornia crassipes, Myriophyllum spicatum, Echinochloa pyramidalis, Typha domingensis and Phragmites australis can survive in extreme conditions and tolerate very high heavy metals concentrations, making them an excellent choice for phytoremediation and biomonitoring programs (FAWZY, BADR, EL-KHATIB & ABO-EL-KASSEM, 2012).
In Guama River, previous studies reported high levels of Ca2+, Mg2+ and Mn2+ on the leaves and fruits of Montrichardia linifera, an aquatic macrophyte popularly known as aninga (AMARANTE, SILVA, MÜLLER & MÜLLER, 2011; AMARANTE, MÜLLER, DANTAS, ALVES, MÜLLER & PALHETA, 2010; AMARANTE, SILVA, SOLANO, NASCIMENTO, MORAES, SILVA &UNO, 2009), indicating that perhaps this plant has the ability to bioaccumulate metals in their biomass and thus, promote phytoremediation. M. linifera belongs to the family Araceae and occurs significantly along the rivers and streams of the Amazon, including River Guama (Figure 1), and serve as food for fishes, turtles, manatees and buffaloes (AMARANTE, MÜLLER, DANTAS, ALVES, MÜLLER & PALHETA, 2010; AMARANTE, SILVA, SOLANO, NASCIMENTO, MORAES, SILVA &UNO, 2009).
The aim of this study was to investigate the levels Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ in different tissues of M. linifera (root, stem, leaf) as well as sediment and surface water, collected in two different areas (at riverbank, with periodic flooding according to the movement of the tides, and at “igapó”, permanently flooded area) in order to ascertain whether there are differences in the composition of these minerals in relation to the collection site, to know which part of the plant these elements are concentrated and calculate the Bioaccumulation Factor (BAF) and Bioconcentration Factor (BCF) as phytoremediation parameters.
Surface water, sediments and aquatic plant Montrichadia linifera were sampled from two sites located in right bank of the River Guama (Figure 2), on the campus of the Federal Rural University of Amazonia, Belem, Para, Brazil. Site I (01º27’53.10’’ S, 048°26’02.90” W and average altitude of 4 m) is at riverbank and affected by irregular disposal of urban waste effluents, with periodic flooding according to the movement of the tides; site II (01°27’42.90” S, 048º26’10.70’’W and average altitude of 10 m) is located 500 m far from site I, toward the mainland, at igapo. All samples (water, sediments, and plants) were collected in triplicates, in three equidistant points on each site, in march/2012, between 8:00 and 10:00 a.m., at low tide time at both sites. In the Amazon, as a rainforest, there is no defined seasons as dry and rain and since these areas are always covered by the tides, regardless of the time of year.
Figure 1
Population of M. linifera in the River Guama. Significant occurrence in the Amazon.

1. Surface sediment (0 – 20 cm) was collected with auger and the samples were packed in sterile plastic bags. The sediments samples were air dried, disaggregated, homogenized and passed through a sieve coarse mesh (2 mm). For determination the metals Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ were determined using 5 g of homogenized sample and the extraction was performed with 25 mL of solution Mehlich 1 (HCl 0.05 mol L-1 + H2SO4 0.0125 mol L-1), followed by stirring for 5 min to 120 rpm. After stirring, the suspension was filtered through Whatman filter paper No. 42 (BOTERO, SOUZA, SANTOS, OLIVEIRA & AMARANTE, 2014).
2. Water samples (500 mL) was collected and stored in sterile bottle, filtered by 1 h of collection using Millipore filter paper type HA 0.45 μm pore size. All samples were kept in amber flasks at 4 °C. Then, aliquots of 25 mL were digested with a mixture of nitric acid (14 mol L-1) and hydrogen peroxide (30% v/v) at a ratio of 3:1 in a digestion block at 150°C for 2 h. After digestion, the samples were filtered and transferred to 50 mL volumetric flasks, measured up to the mark with deionized water.
3. Adult plants were selected for plant material collect. M. linifera was identified by Drª Alba Lins (Coordination of Botany of the Emilio Goeldi Museum) and an exemplar was deposited in the Herbarium João Murça Pires (MG 204905). Plant material was separated in leaves, stem and roots. The leaves were separated in two parts, “petiole+sheath” and lamina. After separation, each part was washed with water, then with deionized water to remove all the debris and other foreign particles and oven-dried (105°C) to constant weight. The dried material was triturated in a knife mill. Approximately 0.3 g of homogeneous samples of leaves (petiole and lamina), stem and roots powder were weighed and digested with a mixture of nitric acid (14 mol L-1) and hydrogen peroxide (30% v/v) at a ratio of 3:1 in a digestion block at 150°C for 2 h. After digestion, the samples were filtered, transferred to 50 mL volumetric flasks and measured up to the mark with deionized water (AMARANTE, SILVA, MÜLLER & MÜLLER, 2011; AMARANTE, MÜLLER, DANTAS, ALVES, MÜLLER& PALHETA, 2010).
For pH measurements in sediment samples were used 10 cm3 of homogenized samples and adding 25 mL of deionized water, followed by stirring. After 1 hour, the system was again stirred and pH was measured (SILVA, 2009). Water samples pH was measured immediately after collection with pH meter.
Metals concentrations in water, sediment and plant samples were determined by a flame atomic absorption spectrophotometer (FAAS AA 904 (Instrumentos Científicos C.G. Ltda.). All the analyses were carried out on three subsamples.
The analytical procedure was checked with tests of addition and recovery of the analytes in samples randomly chosen. The samples were fortified by additions of known concentrations of the analytes and the percent recovery (Rec %) was determined according to the formula I:
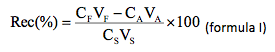
wherein: CF concentration of the analyte in the sample fortified; VF volume of sample of the recovery test (VA + VS); CA concentration of the analyte in the sample not-fortified; VA volume of sample used in the recovery test; CS concentration of standard of the analyte used to fortify the sample; VS volume of standard of the analyte used in the recovery test.
Further, to verify the accuracy of the method, the same digestion method of plant dry matter was applied to Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ determination in certified reference material, Poplar leaves powder GBW 07604 of National Research Center for Certified Reference Materials (CRMs, Beijing, China).
In the present investigation, the bioaccumulation factor (BAF) was calculated using the formula II outlined by SADIQ (1992) and the bioconcentration factor (BCF) was calculated using the formula III according to ESRINGÜ & TURAN (2012):

Results of analytes concentrations in water and sediment samples, in relation to the collection site, were subjected to analysis of variance (F test).
To further verify the accuracy of the method, the developed method was applied to the determination of Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ in certified reference materials of poplar leaves GBW 07604, and the analytical results are given in Table 1 with the limit of detection (LOD) and limit of quantification (LOQ). The obtained values by the proposed method fitted very well with certified values.
Table 1
Analytical results for Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ in
certified reference material GBW07604 (poplar leaves) and limit of
quantification (LOQ) and limit of detection (LOD).
Sample |
Element |
Measured value * (µg g-1) |
Certified value (µg g-1) |
GBW07604 |
Ca2+ |
18093.00 ± 69.87 |
18100 |
Mg2+ |
6498.00 ± 29.86 |
6500 |
|
Fe3+ |
270.97 ± 1.15 |
274 |
|
Cu2+ |
9.37 ± 0.12 |
9,30 |
|
Zn2+ |
36.94 ± 0.27 |
37 |
|
Mn2+ |
45.04 ± 0.21 |
45 |
*Values expressed as mean±s (n = 3). LOD: Ca2+- 23.17 µg L-1; Mg2+ - 20.11 µg L-1; Fe3+ - 2.13 µg L-1; Cu2+ - 5.21 µg L-1; Zn2+ - 2.04 µg L-1; Mn2+ - 3.77 µg L-1. LOQ: Ca2+- 69.88 µg L-1; Mg2+ - 99.16 µg L-1; Fe3+ - 11.05 µg L-1; Cu2+ - 30.08 µg L-1; Zn2+ - 11.12 µg L-1; Mn2+ - 27.87 µg L-1.
The water and sediment pH in two sites were similar, water pH were 6.41 and 6.17, and sediment pH were 5.04 and 5.02 at river bank and igapo respectively, indicating that both systems are acids.
The results for concentrations of Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ in sediment samples, water and different tissues of Montrichardia linifera collected in two different sites, riverbank and igapo, are presented in Table 2.
Table 2
Concentrations of Ca2+, Mg2+, Fe3+, Cu2+, Zn2+ and Mn2+ in
water, sediment and Montrichardia linifera organs collected in
different sites (riverbank and igapo).
Metals |
RIVERBANK |
||||||||
Leaf |
|||||||||
Water (mg L-1) |
Sediment (mg kg-1) |
Root (mg kg-1) |
Stem (mg kg-1) |
Petiole+Sheath (mg kg-1) |
Lamina (mg kg-1) |
||||
Ca2+ |
1322.12 ± 110.00 |
2013.45 ± 18.03 |
6063.33 ± 198.70 |
3725.13 ± 324.49 |
26791.13 ± 805.59 |
12165.70 ± 402.11 |
|||
Mg2+ |
778.33 ± 111.55 |
747.08 ± 93.55 |
1415.17 ± 329.99 |
131.04 ± 2.21 |
3185.27 ± 194.81 |
2523.12 ± 81.89 |
|||
Fe3+ |
4.33 ± 0.04 |
797.87 ± 42.41 |
17369.57 ± 1764.61 |
152.79 ± 17.69 |
521.72 ± 56.30 |
164.05 ± 9.66 |
|||
Cu2= |
< LD |
3.63 ± 0.10 |
< LD |
< LD |
< LD |
< LD |
|||
Zn2+ |
< LQ |
8.7 ± 0.53 |
76.05 ± 2.98 |
22.19 ± 3.39 |
41.88 ± 1.82 |
36.29 ± 1.61 |
|||
Mn2+ |
0.54 ± 0.03 |
206.89 ± 7.93 |
1443.77 ± 24.75 |
221.60 ± 3.48 |
1445.71 ± 54.20 |
1871.89 ± 21.86 |
|||
|
IGAPÓ |
||||||||
Ca |
2081.66 ± 320.00 |
1793.65 ± 43.28 |
10010.98 ± 310.14 |
5725.48 ± 716.57 |
14674.60 ± 504.34 |
8622.30 ± 700.04 |
|||
Mg |
710.00 ± 60.00 |
708.65 ± 38.62 |
1670.73 ± 47.56 |
77.87 ± 4.23 |
2021.13 ± 70.48 |
1988.27 ± 102.54 |
|||
Fe |
9.09 ± 0.27 |
735.87 ± 35.90 |
8161.68 ± 163.84 |
364.47 ± 75.19 |
82.41 ± 7.31 |
83.77 ± 16.05 |
|||
Cu |
< LOD |
2.80 ± 0.25 |
< LOD |
< LOD |
< LOD |
< LOD |
|||
Zn |
< LOQ |
12.35 ± 0.53 |
250.75 ± 13.51 |
192.00 ± 17.27 |
378.75 ± 39.03 |
525.75 ± 44.42 |
|||
Mn |
0.78 ± 0.01 |
431.93 ± 1.35 |
365.02 ± 0.23 |
515.95 ± 20.13 |
1509.69 ± 117.46 |
1914.18 ±42.67 |
|||
LOD = Limit of detection; LOQ = Limit of quantitation. Values expressed as mean±standard deviation(n = 3)
The concentrations of the studied metals in the sediment and water have the same trend in the two sites, in sediment samples Ca2+ > Fe3+ > Mg2+ > Mn2+ > Zn2+ > Cu2+ and in water samples Ca2+ > Mg2+ > Fe3+ > Mn2+. In the water samples, the elements Cu2+ and Zn2+ showed to be below the limits of detection (LODCu2+ = 67.19 μg L-1) and quantitation (LOQZn2+ = 106.01 μg L-1), respectively, in both sites.
The results expressed by analysis of variance (ANOVA) showed that there are differences between the sampling sites in the concentration of Ca2+, Mg2+, Fe3+ and Mn2+ in the water (p <0.05) and Ca2+, Cu2+, Zn2+ and Mn2+ in the sediment (p <0.01) (Table 3). However, for concentrations of Fe3+ and Mg2+ in the sediment, and Zn2+ in the water there was no significant variation in relation to the collection sites.
Table 3.
F values (ANOVA) of the nutrients concentration in the
water and in the sediment in relation to the collection sites.
Variable |
|
Ca |
Mg |
Fe |
Cu |
Zn |
Mn |
Site |
Water |
14.8* |
13500.6* |
885.2* |
- |
0.12ns |
5769.8* |
Sediment |
65.95** |
0.43 ns |
3.73 ns |
27.94** |
70.24** |
2349.76** |
*p < 0.05; ** p < 0.01; ns not significant
In the plant, the elements Ca2+, Mg2+ and Mn2+ were concentrated in the leaves. Practically twice of Ca2+ is deposited on the region "petiole + sheath" (riverbank = 26791.13 ± 805.59 mg kg-1; igapo = 14674.60 ± 504.34 mg kg-1) compared to the leaf lamina (riverbank = 12165.70 ± 402.11 mg kg-1; igapo = 8622.30 ± 700.04 mg kg-1) (Table 2). High Ca2+ and Mg2+ concentrations were expected, compared to the other elements analyzed, since they are macronutrients. However, for Mn2+ (a trace element) the high levels found in the plant can be justified by the fact that the soil remains at long time flooded, and as the concentration of manganese in the soil solution increases after the submersion of the soil (MATTAR, VIEIRA & SILVA, 2002), due to the reduction process occurs greater availability of manganese in the soil and increased plant uptake (IWATA, 1975; ABREU, FERNANDES, MARTINS & RODRIGUES, 2006).
Furthermore, studies in soil samples from the right bank of Guama River, on the Universidade Federal Rural da Amazonia (UFRA) next to the study research’s area, showed pH values < 5 of acidic soils (LOPES, FERNANDES, GRIMALDI, RUIVO, RODRIGUES & SARRAZIN, 2006). The high acidity was also found in the same study area at different times of the year, regardless of the period that the flood occurs (ABREU, FERNANDES & RUIVO, 2007). In general, acidic pH conditions favor toxic concentrations accumulation of manganese, due to the increased solubility at pH 5.0 (FOY, 1973; VELOSO, MURAOKA, MALAVOLTA & CARVALHO, 1995). The highest Fe3+ concentrations were found in the roots on both sites, the riverbank (17369.57 ± 1764.61 mg kg-1) and igapo (8161.68 ± 163.84 mg kg-1) (Table 2). Cu2+ was no detected in any plant part, as results for this element were all below the detection limit (LOD = 25.91 μg L-1).
Zn2+ was the exception, once presented higher concentrations in the samples from the igapo, ranging from 192.00 to 525.75 mg kg-1, compared to the riverbank where the variation was from 22.19 to 76, 05 mg kg-1 (Table 2). In fact, the content of Zn2+ on igapo sediment (12.35 mg kg-1) was higher than on the riverbank sediment (8.7 mg kg-1) and this difference may be related to the fact that the soil of igapo is permanently flooded, unlike the soil of the riverbank that is periodically flooded, and influenced by tidal movement. In acid soils of pH < 5.5 and anaerobes, as is the case of the igapo in this study, the nitrification rate is very low, which results in accumulation of ammonia (NH3) (ABREU, FERNANDES & RUIVO, 2007; SILVA, VALE, ANDERSON & KOBAL, 1999). Although Zn2+ is a low mobility cation, strongly adsorbed on metal oxides and clay, also can form insoluble sulfides (GUILHERME, MARQUES, PIERANGELI, ZULIANI, CAMPOS & MARCHI, 2005). Once on the environment can form complexes with ammonia and may be remobilized off the sediment for form these complexes and therefore get available to the plant (FATMA, 1999).
The distribution of this element in the plant was also different. At igapo the highest Zn2+ content was found in the leaf (525.75 mg kg-1) while on the riverbank the highest content was found in the root (76.05 mg kg-1) (Table 4). Possibly, the anaerobic conditions of igapo soil (permanently flooded) allow the plant to absorb more Zn2+ than the plant that inhabits the riverbank and is exposed to tidal movement.
In Table 4 are shown the percentages of each element found in the system (water-sediment-plant), and it's possible to map how M. linifera stocks these nutrients in their biomass. Among all the Ca2+ from riverbank, over 90% was stored in the biomass of M. linifera and only 2.54% in the water and 3.87% in the sediment. 74.80% were stored in leaves. Also on igapo 90% of Ca2+ was in biomass and of this amount, 54.29% was found in the leaves, 4.85% in the water and 4.18% in the sediment. The Mg2+ behavior was similar in both collection sites, approximately 80% of total was found in the biomass. On average 60% was present in the leaves and just about 9 - 10% in the water and in the sediment, respectively.
Of all the Fe3+ found in the systems (riverbank and igapo), 95.77% and 92.10% was in the biomass, respectively, where a percentage of 91.37% and 86.48% was stored in the roots and only 0,02 - 0.10% in the water and 4.20 - 7.80% in the sediment, respectively (Table 4).
Mn2+ also showed the same storage tendency, in both collection sites, i.e., more than 90% were present in the biomass, and only about 0.01 - 0.02% in the water and 4 - 9% in the sediments. In the biomass, about 60 to 70% of Mn2+ was also found in the leaves (Table 4).
The Mn2+ concentrations obtained from the leaves of M. linifera in this study were similar to those obtained in a previous study (AMARANTE, SILVA, SOLANO, NASCIMENTO, MORAES, SILVA & UNO, 2009), around 3000 mg kg-1 (Table 5).
In relation to Cu2+ in the systems, it was found only in sediments from both collection sites. Indeed, in other studies, this element also showed lower concentrations not only in species of Montrichardia, but also in other aquatic plants, such as some species of Eichhornia, Pistia and Salvinia, as shown in Table 5.
The exception was Zn2+, although presented a percentage more than 95% of storage in the biomass of both collection sites, it was observed that in the sampling from the riverbank there was a division of this element, where a percentage of 41.08% was stored in the root and almost the same percentage, 42.22%, was stored in the leaf. Different behavior was observed in igapo samples, where only 18.44% was stored in the root while a higher percentage (66.53%) was stored in leaves (Table 4). That in the igapo, the contact of the plant with the soil solution is full time, and so may favor Zn2+ accumulation in the leaves, contrary to what occurs on the riverbank.
The stem was the tissue of the plant, with the lowest concentrations of all elements, according to the following pattern Ca2+ > Mn2+ > Fe3+ > Mg2+ > Zn2+ for the plant collected on the riverbank and Ca2+ > Mn2+ > Fe3+ > Zn2+ > Mg2+ for igapo samples, as shown in Table 2.
Phytoremediation parameters calculated in terms of the Bioaccumulation Factor (BAF) and Bioconcentration Factor (BCF) from the total biomass of M. linifera are shown in Table 6 and reveal that this plant has high potential to bioaccumulate Mn2+ (9227.72 mg kg-1 and 5519.03 mg kg-1) and Fe (4205.11 mg kg-1 and 956.25 mg kg-1) of both systems, riverbank and igapo respectively, and considerable capacity to bioaccumulate Zn2+ in igapo system (109.08 mg kg-1) compared to the riverbank (20.27 mg kg-1).
In general, this plant accumulates more elements at riverbank than igapo (exception for Zn) (Table 4). Thus, the results show that M. linifera concentrate almost 1.67 times more Mn2+ and 4.39 times more Fe3+ in the riverbank that in the igapo, which in turn accumulates 5.38 times more Zn2+ than on the riverbank.
Table 4
Percentage (%) of elements Ca2+, Mg2+, Fe3+, Cu2+, Zn2+
and Mn2+ in the system water-sediment-M. linifera
Elements (%) |
Ca2+ |
Mg2+ |
Fe3+ |
Cu2+ |
Zn2+ |
Mn2+ |
|||||||
Samples / Local collection |
RB |
IG |
RB |
IG |
RB |
IG |
RB |
IG |
RB |
IG |
RB |
IG |
|
Water |
2.54 |
4.85 |
8.86 |
9.89 |
0.02 |
0.10 |
< LOD |
< LOD |
< LOD |
< LOD |
0.01 |
0.02 |
|
Sediment |
3.87 |
4.18 |
8.51 |
9.87 |
4.20 |
7.80 |
100 |
100 |
4.70 |
0.91 |
3.99 |
9.12 |
|
Root |
11.64 |
23.33 |
16.12 |
23.28 |
91.37 |
86.48 |
< LOD |
< LOD |
41.08 |
18.44 |
27.82 |
7.70 |
|
Stem |
7.15 |
13.34 |
1.49 |
1.09 |
0.80 |
3.86 |
< LOD |
< LOD |
11.99 |
14.12 |
4.27 |
10.89 |
|
Leaf |
Petiole+Sheath |
51.44 |
34.20 |
36.28 |
28.16 |
2.74 |
0.87 |
< LOD |
< LOD |
22.62 |
27.86 |
27.85 |
31.87 |
Lamina |
23.36 |
20.09 |
28.74 |
27.70 |
0.86 |
0.89 |
< LOD |
< LOD |
19.60 |
38.67 |
36.06 |
40.40 |
|
Total |
74.80 |
54.29 |
65.02 |
55.86 |
3.60 |
1.76 |
< LOD |
< LOD |
42.22 |
66.53 |
63.91 |
72.27 |
|
Total in the Biomass |
93.59 |
90.96 |
82.63 |
80.23 |
95.77 |
92.10 |
< LOD |
< LOD |
95.29 |
99.09 |
96.00 |
90.86 |
|
RB = Riverbank. IG = Igapó. Value in bold = the highest percentage found in the plant organ. LOD = Limit of detection
-----
Table 5
Concentration values (mg kg-1) of Ca2+, Mg2+, Fe3+, Cu2+, Zn2+
and Mn2+ in M. linifera obtained in this work and compared with
other aquatic plants.
Species |
Part of plant |
Concentrations (mg kg-1) |
References |
|||||
Ca2+ |
Mg2+ |
Fe3+ |
Cu2+ |
Zn2+ |
Mn2+ |
|||
Montrichardia linifera* |
Total biomass RB |
48745.29 |
7254.60 |
18208.13 |
<LOD |
176.41 |
4982.97 |
This work |
Total biomass IG |
39033.36 |
5758.00 |
8692.33 |
<LOD |
1347.25 |
4304.84 |
||
Leaf RB |
38956.83 |
5708.39 |
685.77 |
<LOD |
78.17 |
3317.60 |
||
Leaf IG |
23296.90 |
4009.40 |
166.18 |
<LOD |
904.50 |
3423.87 |
||
Montrichardia linifera* |
Leaf RS |
19150.00 |
5730.00 |
70.79 |
7.08 |
272.02 |
3279.46 |
[1] |
Leaf LRS |
31530.00 |
9750.00 |
58.82 |
6.81 |
255.90 |
3612.23 |
||
Montrichardia arborescens* |
Leaf |
18500.00 |
4620.00 |
1150.00 |
<LOD |
259.00 |
<LOD |
[2] |
Eichhornia crassipes Pistia stratiotes* |
Total biomass |
15100.00 10900.00 |
3920.00 2150.00 |
5425.00 1391.67 |
25.83 9.17 |
81.83 29.83 |
1233.33 2145.83 |
[3] |
Eichhornia crassipes |
Leaf |
30200.00 |
<LOD |
<LOD |
<LOD |
<LOD |
<LOD |
[4] |
Anona sp. |
Leaf |
14400.00 |
3200.00 |
<LOD |
<LOD |
<LOD |
<LOD |
|
Eichhornia crassipes Pistia stratiotes* Salvinia auriculata |
Total biomass |
11550.00 22350.00 9180.00 |
2930.00 4500.00 2480.00 |
4671.33 4050.00 3206.00 |
9.75 8.58 11.14 |
187.00 233.27 250.33 |
1254.00 1319.00 1491.00 |
[5] |
Egeria densa Egeria najas Ceratophyllum demersum |
Total biomass |
17300.00 16500.00 11800.00 |
3600.00 7600.00 9800.00 |
2154.90 2958.50 3526.20 |
5.90 5.70 4.60 |
102.90 130.50 149.00 |
<LOD <LOD <LOD |
[6] |
Legend: * Species of Araceae family; RB = Riverbank. IG = Igapó. RS = Rainy Season; LRS = Less Rainy Season
[1] , [5] AMARANTE, SILVA, MÜLLER & MÜLLER, 2011;
[2] BUSETTI, RUIVO, SALES & BERRÊDO, 2009;
[3] HENRY-SILVA &CAMARGO, 2006;
[4] PORTAL, LIMA, LUZ & BATAUS, 2002;
[6] CORRÊA, VELINI & ARRUDA, 2003.
-----
Table 6
Bioaccumulation Factor (BAF) and Bioconcentration Factor (BCF) of Ca2+,
Mg2+, Fe3+, Cu2+, Zn2+ an Mn2+ in the total biomass of M. linifera to the
riverbank (RB) and igapó (IG)
|
Elements |
||||||||||||||||
Site |
Cu2+ |
|
Zn2+ |
|
Mn2+ |
|
Fe3+ |
|
Ca2+ |
|
Mg2+ |
||||||
BAF |
BCF |
BAF |
BCF |
BAF |
BCF |
BAF |
BCF |
BAF |
BCF |
BAF |
BCF |
||||||
RB |
nd |
nd |
nd |
20.27 |
9227.72 |
24.09 |
4205.11 |
22.82 |
36.87 |
24.21 |
9.32 |
9.71 |
|||||
IG |
nd |
nd |
nd |
109.08 |
5519.03 |
9.97 |
956.25 |
11.81 |
18.75 |
21.76 |
8.11 |
8.13 |
|||||
nd = not determined. Value in bold = the highest value in the site
The bioaccumulation factor of M. linifera for Mn2+ is 1.67 times greater in the riverbank that in the igapo and this element is concentrated on the leaves, more precisely in the region of "petiole + sheath". The Fe3+ is deposited in the roots and is bioaccumulated 4.4 times more in the riverbank that in the igapo. Moreover, this plant can concentrate more Zn2+ in the environment of igapo, around 5.38 times more than in the riverbank. In igapo, Zn2+ is equally stored in the roots and leaves, while on the riverbank the highest concentration was found on the leaves. M. linifera can tolerate very high concentrations of metals which make them an excellent choice for phytoremediation and biomonitoring studies.
The authors thank to the CNPQ/MCTIC (National Council for Scientific and Technological Development / Ministry of Science, Technology, Innovation and Communications) and CAPES / MEC (Coordination of Improvement of Higher Level Personne / Ministry of Education) for the scholarships granted to the students.
ABREU, E. M. A.; FERNANDES, A. R.; MARTINS, A. R. A.; RODRIGUES, T. E. (2006); Forage production and nutritive value of forage species under grazing conditions in low floodplain soil of the Guama River, Acta Amazonica, 36, 11-18.
ABREU, E. M. A.; FERNANDES, A. R.; RUIVO M. L. P. (2007); Variação temporal e vertical de atributos químicos de um gleissolo do Rio Guamá cultivado com Canaranas, Revista Brasileira de Ciências do Solo, 31, 277.
AMARANTE, C. B.; MÜLLER, R. C. S.; DANTAS, K. G. F.; ALVES, C. N.; MÜLLER, A. H.; PALHETA, D. C. (2010); Chemical composition and nutritional value of leaves and fruits of aninga (Montrichardia linifera, Araceae) for large herbivores, Acta Amazonica, 40, 729-736.
AMARANTE, C. B.; SILVA, J. C. F.; MÜLLER, R. C. S.; MÜLLER, A. H. (2011); Evaluation of mineral composition of tea from senescent leaf of Montrichardia linifera (Arruda) Schott (Araceae) by flame atomic absorption spectrometry (FAAS), Quimica Nova, 34, 419-423.
AMARANTE, C. B.; SILVA, J. C. F.; SOLANO, F. A. R.; NASCIMENTO, L. D.; MORAES, L. G.; SILVA, F. G.; UNO, W. S. (2009); Estudo Espectrométrico das folhas da aninga (Montrichardia linifera) coletadas à margem do Rio Guamá no campus da UFPA, Belém-PA. Uma contribuição ao estudo químico da família Araceae, Revista Cientifica UFPA, 7, 1-19.
BOTERO, W. G.; SOUZA, S. O.; SANTOS, O. S.; OLIVEIRA, L. C.; AMARANTE, C. B. (2014); Influence of sediment humic substances on the bioavailability of metals in the aquatic system, Quimica Nova, 37, 943-949.
BUSETTI, E. P. C.; RUIVO, M. L.; Sales, M. E.; BERRÊDO, J. F. (2006); Belém de água e ilhas, 1st ed.; Cejup:Belém. [2]
CORRÊA, M. R.; VELINI, E. D.; ARRUDA, D. P. (2003); Planta Daninha, 21, 7. [6]
Environmental Foundation of Santa Catarina – FATMA (1999); Relevance of quality parameters of the water applied to running water. Part I: General characteristics, nutrients, trace elements and inorganic harmful substances, biological, FATMA / GTZ: Florianopolis, BR.
ESRINGÜ, TURAN, A.; M. (2012); The Roles of Diethylenetriamine Pentaacetate (DTPA) and Ethylenediamine Disuccinate (EDDS) in Remediation of Selenium from Contaminated Soil by Brussels Sprouts (Brassica oleracea var. gemmifera), Water, Air, Soil Pollution, 223, 351-362.
FAWZY, M. A.; BADR, N. E.; EL-KHATIB, A.; ABO-EL-KASSEM, A. (2012); Heavy metal biomonitoring and phytoremediation potentialities of aquatic macrophytes in River Nile, Environmental Monitoring and Assessment, 184, 1753-1771.
FIGUEIRA, R.; RIBEIRO, T. (2005); Transplants of aquatic mosses as biosorbent of metals released by a mine Zuent, Environmental Pollution, 136, 293-301.
FOY, C. D. (1973); Manganese, National Academy of Science. Washington DC.
GUILHERME, L. R. G.; MARQUES, J. J.; PIERANGELI, M. A. P.; ZULIANI, D. Q.; CAMPOS, MARCHI, M. L.; G.(2005); Tópicos em ciência do solo, Sociedade Brasileira de Ciência do Solo, Viçosa, BR.
HENRY-SILVA, G. G.; CAMARGO, A. F. M. (2006); Planta daninha, 24, 21.
IBGE. Censos demográficos 1960, 1970, 1980, 1991, 2000 e 2010.
IWATA, T. (1975); Studies on the occurrence of tomebagare a newly found physiological disease of Rice and its peventive measures, Fukui Agricultural Experiment Station, 6, 1-66.
KHAMBHATY, Y.; MODY, K.; BASHA, S.; JHA, B. (2009); Biosorption of chromium (VI) onto marine Aspergillus niger: experimental studies and pseudosecond order kinetics, World Journal of Microbiology and Biotechnology, 25, 1413-1421.
KUYUCAK, N.; VOLESKY, B. (1988); Biosorbents for recovery of metals from industrial solutions, Biotechnology Letter, 10, 137-142.
LOPES, E. L. N.; FERNANDES, A. R.; GRIMALDI, C.; RUIVO, M. L. P.; RODRIGUES, T. E.; SARRAZIN, M. (2006); Características químicas de um Gleissolo sob diferentes sistemas de uso, nas margens do rio Guamá, Belém, Pará, Boletim do Museu Paraense Emilio Goeldi Ciencias Naturais 2006, 1, 127-137.
MARTINS, D.; COSTA, N. V.; TERRA, M. A.; MARCHI, S. R.; VELINI, E. D. (2003); Planta daninha, 21, 21. [5]
MATTAR, R. M. V. C.; VIEIRA, I. S.; SILVA, G. R. (2002); Efeito da inundação sobre o pH e a disponibilidade de fósforo, sódio, ferro e manganês em Gley Pouco Húmico coletado na várzea do Rio Guamá. Belém-PA, Revista Ciencias Agrarias, 37, 113-157.
MERCÊS, S.; BASTOS, A. P. V. (2011); Análise populacional da Região Metropolitana de Belém e do Estado do Pará, 2000-2010, Boletim Informativo Observatório das Metrópoles, 2011. Recuperado de: http://www.observatoriodasmetropoles.net/download/Censo2010_RMB_2011.pdf
PORTAL, R. R.; LIMA, M. A. S.; LUZ, V. L. F.; BATAUS, Y. S. L. (2002); Ci. Anim. Bras., 3, 11.
RIBEIRO, K. T. S. (2004); Água e saúde humana em Belém, Cejup, Belem, BR.
SALT, D. E.; SMITH, R. D.; RASKIN, I. (1998); Phytoremediation, Annual Review of Plant Physiology and Plant Molecular Biology, 49, 643-668.
SADIQ, M. (1992); Toxic metal chemistry in marine environments, Marcel Dekker, New York, US.
SANTOS, M. L. S.; HOLANDA, P.; PEREIRA, I.; RODRIGUES, S.; PEREIRA, MESQUITA, A. R. K. (2014); Influência das Condições da Maré na Qualidade de Água do Rio Guamá e Baia do Guajará. Boletim Técnico-Cientifico do Cepnor , 14, 17 - 25.
SERUDO, R. L.; OLIVEIRA, L. C.; ROCHA, J. C.; PATERLINI, W. C.; ROSA, A.; SILVA, H. C.; BOTERO, W. G. (2007); Reduction capability of soil humic substances from the Rio Negro basin, Brazil, towards Hg(II) studied by a multimethod approach and principal component analysis (PCA), Geoderma, 138, 229-236.
SILVA, C. A.; VALE, F. R., ANDERSON; S. J., KOBAL, A. R. (1999); Pesquisa Agropecuaria Brasileira, 34, 1679.
SILVA, F. C. (2009); Manual de Análises Químicas de Solos, Plantas e Fertilizantes, Embrapa, Brasilia, BR.
VELOSO, C. A. C.; MURAOKA, T.; MALAVOLTA, E.; CARVALHO, J. G. (1995); Influence of manganese on mineral nutrition and growth of the kingdom pepper (Piper nigrum L.), Scientia Agricola, 52, 376-383.
VERMA, V. K.; GUPTA, R. K.; RAI, J. P. N. (2005); Biosorption of Pb and Zn from pulp and paper industry effluent by water hyacinth (Eichhornia crassipes), Journal of Scientific & Industrial Research, 64, 778-781.
1. PhD in Chemistry, Senior Technologist, Coordinator of the Laboratory of Chemical Analysis of the Emílio Goeldi Museum (MPEG), Professor of the Postgraduate Program in Biodiversity and Biotechnology of the Legal Amazon (BIONORTE Network) – Belem, PA, Brazil. e-mail: cbamarante@museu-goeldi.br
2. PhD in Agronomy: Soils and Plant Nutrition, Titular Professor at the Federal Rural University of Amazonia (UFRA) – Belem, PA, Brazil. e-mail: antonio.fernandes@ufra.edu.br
3. PhD student of the Postgraduate Program in Agronomy of the Federal Rural University of Amazonia (UFRA) – Belem, PA, Brazil. e-mail: josemar.jmv@gmail.com
4. Master in Agronomy: Soils and Plant Nutrition, Professor of the Federal Institute of Education, Science and Technology of Amapa (IFAP) – Porto Grande, AP, Brazil. e-mail: nilvan.melo@ifap.edu.br
5. PhD in Agronomy: Soil Physics. Professor at the Federal Rural University of Amazonia (UFRA)– Capanema, PA, Brazil. e-mail: dan_agronomia@hotmail.com
6. PhD in Botany. Associate Researcher of Emílio Goeldi Museum (MPEG) – Belem, PA, Brazil. e-mail: lins@museu-goeldi.br
7. Master in Phytotechnology, PhD student of the Postgraduate Program in Biodiversity and Biotechnology of the Legal Amazon (BIONORTE Network) – Belem, PA, Brazil. e-mail: rjunior@museu-goeldi.br
8. Mechanical Engineer. Department of Economic Development of Pará Mining and Energy (SEDEME). Professional Collaborator of the Emílio Goeldi Museum (MPEG) – Belem, PA, Brazil. e-mail: lourival.ribeiro@sedeme.com.br
9. Civil Engineer. Ex-Officer of the Brazilian Army. Geotechnical expertise. Professional Collaborator of the Emílio Goeldi Museum (MPEG) – Belem, PA, Brazil. e-mail: fadecgoncalves@gmail.com
10. PhD in Chemistry. Adjunct Professor of the Federal University of Alagoas (UFAL) – Arapiraca, AL, Brazil. e-mail: wanderbotero@gmail.com
11. Graduation Student of Mechanical Engineering. (IESAM). Scientific Initiation Program of the Emílio Goeldi Museum (MPEG) – Belem, PA, Brazil) – Belém, PA, Brasil.e-mail: amaralels@outlook.com
12. Graduation Student of Chemistry Bachelor's Degree(UFPA). Scientific Initiation Program of the Emílio Goeldi Museum (MPEG) – Belem, PA, Brazil) – Belém, PA, Brasil.e-mail: andersonbotelho10@hotmail.com