

Vol. 39 (Number 18) Year 2018 • Page 18
Zhanat UZDENBAYEVA 1; Yerlan ANDASBAYEV 2; Ayazhan AYAGANOVA 3
Received: 04/01/2018 • Approved: 26/01/2018
ABSTRACT: The paper is concerned with industrial wastewater cleaning from heavy metals by natural sorbents. The author developed a method of preparing acid-alkali activated bentonite with high sorption capacity in the form of suspensions and pastes. The experimental method was used to obtain a mathematical model: complete factorial analysis at three levels, the equations that describe the impact of all factors studied are derived. |
RESUMEN: El documento trata de la limpieza de aguas residuales industriales a partir de metales pesados mediante absorbentes naturales. El autor desarrolló un método para preparar bentonita activada por ácido alcalino con alta capacidad de sorción en forma de suspensiones y pastas. El método experimental se utilizó para obtener un modelo matemático: análisis factorial completo en tres niveles, se derivan las ecuaciones que describen el impacto de todos los factores estudiados. |
The presence of large deposits of montmorillonite bentonite clays and zeolites allows treating them as the sources of high-quality mineral resources for the production of effective sorbents for the purification of industrial wastewater. This is vital for the Kazakhstan environmental improvement, especially in the mining, ore dressing and metallurgical industrial centers of the Rudny Altai [1, 2 ].
Natural sorbents have a sorption capacity relative to the polar substances: water, alcohols, amine; as well as a high cation exchange capacity.
Among the unique physical and chemical properties of bentonite in natural conditions of its occurrence are the high dispersion that increases hydrophilicity; the presence of colloid-disperse minerals and sol-gel phases that determine the adhesive properties and plasticity of these species as a natural binding materials.
Some natural sorbents are quite active in the natural сondition, but most of them require being activated by chemical or thermal means to increase and regulate their porous structure, changes in the chemical nature of the surface.
One of the largest deposits of bentonite montmorillonite clays is Tagansky deposit in East Kazakhstan.
The bentonites of Tagansky deposit according to geotechnical classification of M.S. Merabishvili and M.M. Kravchenko were divided into the following lithological horizons:
Horizon 10 - "clay soil", rich in humus, water-soluble iron compounds, silica, organic residues;
Horizon 11- "geochemical barrier" consisting of carbonates of calcium, magnesium, opal, zeolite;
Horizon 12 - "waxy" bleached argillite located directly under the geochemical carbonate-alkaline barrier "breastplate";
Horizon 13 - "spotty" clay, saturated with clusters of mineral ferri-jarosite and psilomelane;
Horizon 14 - "mother" montmorillonite clay and montmorillonite-kaolinite- halloysite. The upper part of the given level (power of 1 to 2.5 m) is rich in soluble organic compounds, so the color becomes black.
Horizon 15 - variegated clays with numerous streaks of quartz-feldspar coarse sand.
One of the most important features of these minerals is the presence of isomorphism, which results in the substitution in the crystal lattice of one cation to another.
Bentonite has a maximum exchange speed (exchange capacity of 80-150 mg / eq per 100 g), which can be explained, firstly, by the maximum dispersion of these minerals, and, secondly, by the availability for the exchange of not only external, but also internal mineral surfaces.
In the montmorillonite clays the exchange is being carried out by exchangeable cations of Ca++, Mg++, H+, K+, NH3+, Na+ the relative change in the value from Са++ to Na+ and exchangeable anions SO42-, Cl-, PO43-, NO3-.
Studies have shown that such methods of cleaning wastes from heavy metals with the use of bentonite without activation, "Magnafloc 10" composite flocculant and bentonite, and thermally activated bentonitane can provide a degree of purification to the level maximum permissible concentration for fishery (MACfish).
The author suggests aluminosilicate acid activation technique consisting in acid activation of bentonite by 20% sulfuric acid and neutralization of the aqueous suspension of Horizon 11 bentonite.
The technology for producing aluminosilicate sorbents with increased sorption capacity includes processing bentonite by using acidic reagents under normal conditions. As the acid reactant the 20% sulfuric acid solution with the pH between 1 and 2 was used, and as the alkaline reagent - an aqueous suspension of Horizon 11 bentonite.
Being transferred in the result of acid-alkaline activation to the extremely non equilibrium state, the activated bentonite has chemically reactive mineral matrix, which, according to the Le Chatelier principle, strives to return to its original stable state. At the same time there is a process of regeneration (reductive mineralization), which is involved with all kinds of reactive contaminants, that leads to their irreversible chemisorptions binding with silica-alumina sorbent. Colloid-dispersion and the sol-gel phase of hydrolyzed aluminosilicate, that contained in sorbent, provides acceleration of flocculation of the dispersed phase of contaminated liquid and suspended solids in it, and colloidal particles followed by their intense. The proposed method allows adding all the required components for cleaning into the water simultaneously. The developed method of cleaning wastewater from heavy metals is focused on the use of cheap natural raw materials in the form of clay rock - aluminosilicates, - which are most environmentally friendly in the environment compared to various chemical reagents.
In the process of the study were used General methods of research: methods of analysis of financial statements: horizontal, vertical, ratio, comparison, and other.
To study the system of industrial waste of Kazakhstan were used General scientific and special research methods:
- review of the regulatory framework;
- analytical method;
- economic-mathematical calculations.
Methods of preparing the acid-alkali activated bentonite that proposed by the author are:
1 20% sulfuric acid in an amount of 100 ml is added to bentonite of Horizons 14 in coma in an amount of 170 g;
2 Acid activation takes place within 24 hours;
3 Separately, an aqueous suspension of Horizon 11 bentonite is prepared. For this purpose, 170 g of bentonite of Horizon 11 is mixed with 100 ml of distilled water;
4 The acid activated bentonite of Horizon 14 is added to an aqueous suspension of Horizon 11 bentonite; this is accompanied by activation of the alkaline sorbent until reaching the pH of 7.5 (water extract).
In the author's research, there was used an activation of sulfuric acid because there is a need in sulfuric acid recycling in the region.
The most effective sorbents for the purification of effluent from the POF of heavy metals are Horizons 14, and 13 bentonite, as there is ferri-jarosite mineral in Horizon 14, which is a constituent of Al2 (SO4) 3iFe2 (SO4)3 coagulants.
By the method of activating active adsorbents for the purification wastewater is produced during activation of bentonite with hot acid for 6 hours.
The sharp increase in activity of acid treated bentonite, as shown, is connected with the substitution of replacement of cations of sodium, calcium, magnesium with H +, A13 + and an increase in total acidity exchange and the surface area.
When the acid activated bentonite except the replacement of exchangeable cations Na +, Ca2 +, Mg2 + for hydrogen ions of activating acid, the crystalline structure of montmorillonite is destroyed. In the exchange position appear H + and A13 + ions. Moreover, the exchange of aluminum ions is considerably greater than that of hydrogen ions. The emergence of A13 + is probably due to the extraction of the A13 + from montmorillonite structure under the action of hydrogen ions of activating acid and binding A13 + with bars to compensate its negative charges. With other conditions being equal, negative charge of neutralization lattice of montmorillonite A13 + is faster compared to the singly and doubly charged cations.
While in acid treatment of bentonite the value of exchange capacity is reduced, the hydrogen ions activating acid not only displace the exchangeable sodium cations, calcium, magnesium exchange positions, but also penetrate into the interior of the montmorillonite structure and replace magnesium ions, iron, aluminum and other metals from the structure. This is accompanied by the destruction of montmorillonite structure, and in the exchange position along with the hydrogen ions appear aluminum cations. Thus a certain amount of silica is released, which leads to a 2-4 fold increase in surface activated samples compared to the surface of natural bentonites. Acid treatment does not change the predominant pore sizes (1.5 nm) of initial bentonite, but gives rise to large pores and transient increase of the sample porosity.
In preparing activated sorbent the aqueous suspension of Horizon 11 bentonite is added, wherein the acid activation of an alkaline sorbent takes place; and as a result there appears mineral of tobermorite - calcium hydro silicat. Calcium silicates formed in the acid-alkali activation are capable of ion exchange. The possibility of isomorphic substitution of Si4 + to Al3 + is typical for bentonite - montmorillonite and calcium silicate.
At acid activation ions of hydrogen and aluminum appear in exchange positions. The montmorillonite structure is destroyed; SiO2 is released, which leads to an increase in available surface for the reacting molecules, and the porosity of sorbent. The process of alkali activation is composed of replacement of cations of Ca2+ by hydrogen ions, while exposure to acid removes impurities and achieved by loosening of the structural skeleton.
It is found that in acidic medium the cation exchange flows at a low rate, as a result of adsorption of hydrogen ions of bentonites by themselves. Cationic exchange on bentonites under alkaline conditions is more active than in the neutral ones, and in the neutral conditions it is stronger than in the acidic. In the treatment by solution of alkali the amount of hydrogen ions exchanged from the bentonites is larger than by treatment with a solution of a neutral salt. The reason is that in the case of bentonite treatment by an alkali solution the calcium ions are replaced by sodium, and hydrogen ions are not only of aluminosilicate complex, and hydrogen ions to hydroxides of silicon and aluminum.
To create a mathematical model was used an experimental method of obtaining a mathematical model of a full factorial analysis at three levels [3].
Plan an experiment to obtain a regression equation in which the response function of Y1, Y2, Y3 will be the degree of purification of effluents from the heavy metals Pb, Zn, Cu, and factors: u1- contact time hours; u2- initial concentration, mg/dm3; u3-mass of sorbent, the resulting equations will determine the individual and combined effect of factors on the degree of purification.
The following factors and ranges of variation were selected for construction of the experimental design (Table. 1).
Table 1
The factors and ranges of variation
Factors |
The value of the factor in the center иi0 |
The interval of variation δi |
u1- contact time, h |
1,5 |
0,5 |
и2 - starting concentration, mg/dm3 |
5 |
2,5 |
и3–mass of sorbents, g |
25 |
5 |
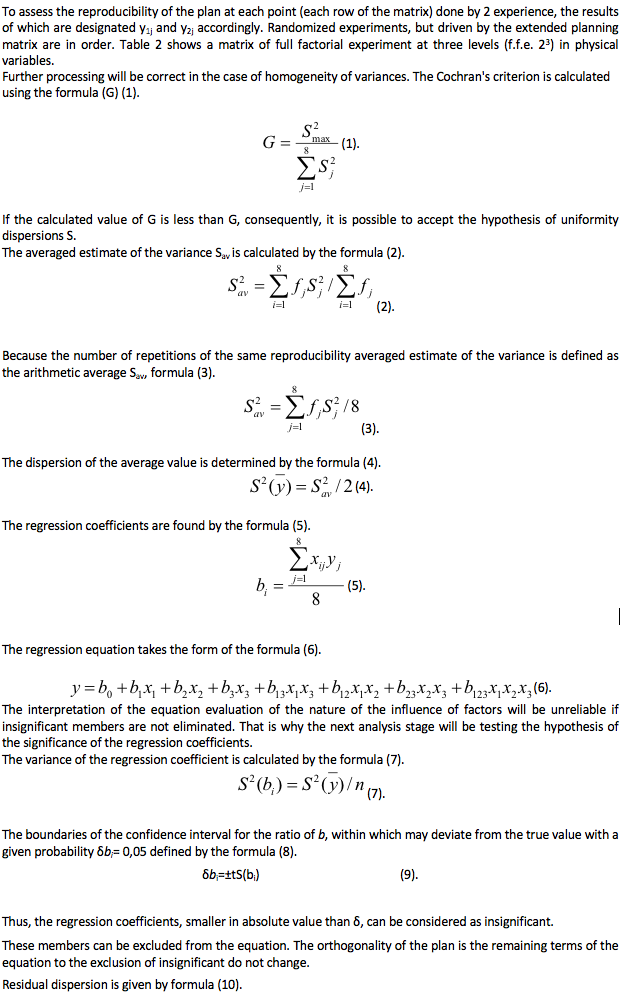


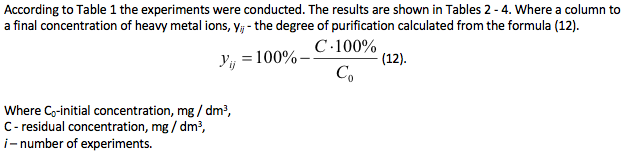
Table 2
The degree of purification of the model solution from Zn2+ ions, experiment No. 1, 2.
-----
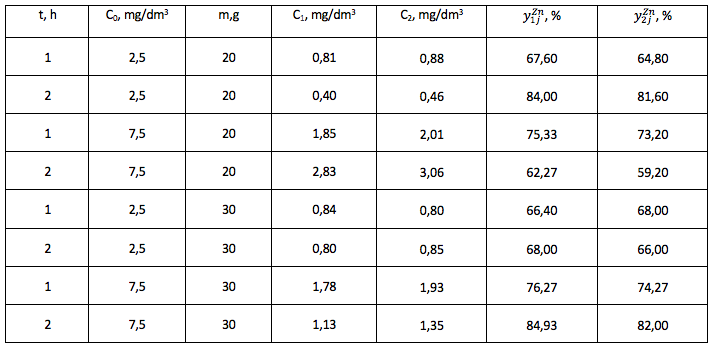
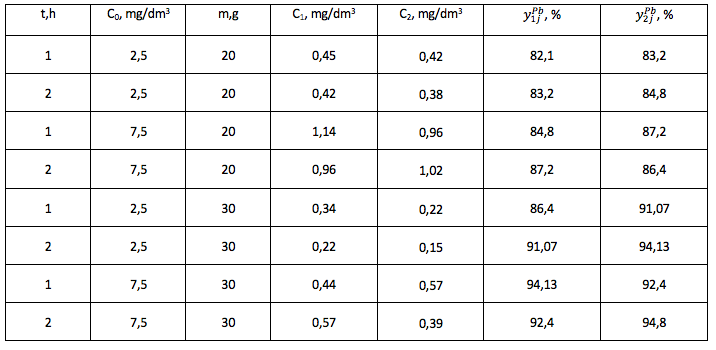
Table 3
The degree of purification of the model solution from Pb2+ ions, experiment No.1, 2.
-----
Table 4
The degree of purification of the model solution from Cu2+ ions, experiments No.1, 2.
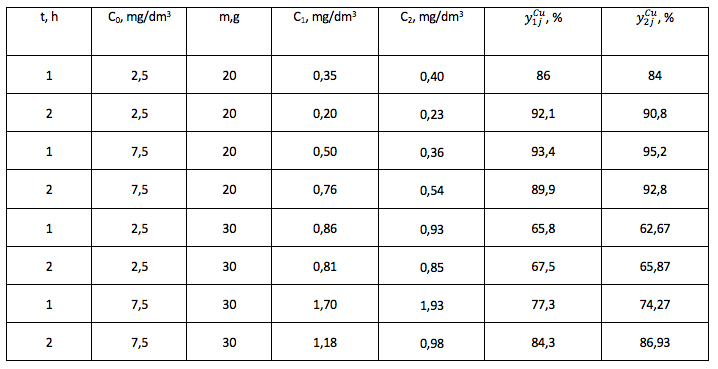
The regression equation for model cleaning solution from the Zinc ions
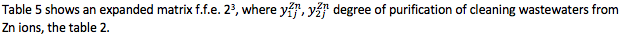
Table 5
Extended matrix of the f.f.e. 23
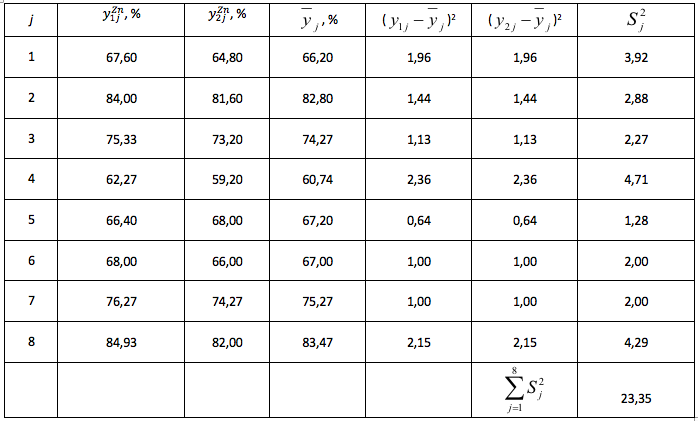
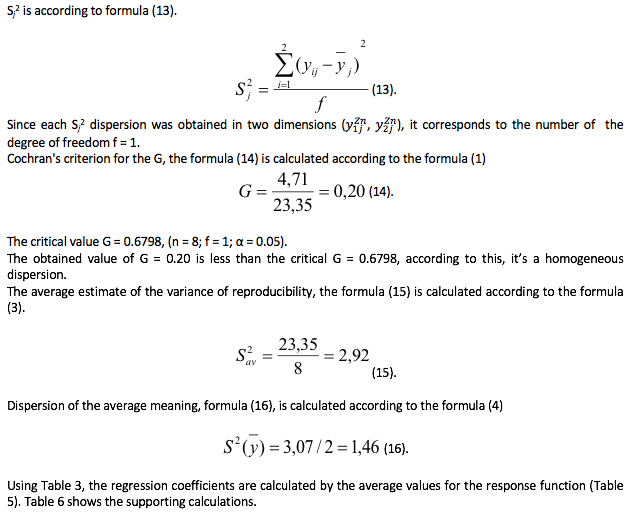
Table 6
Supplementary table
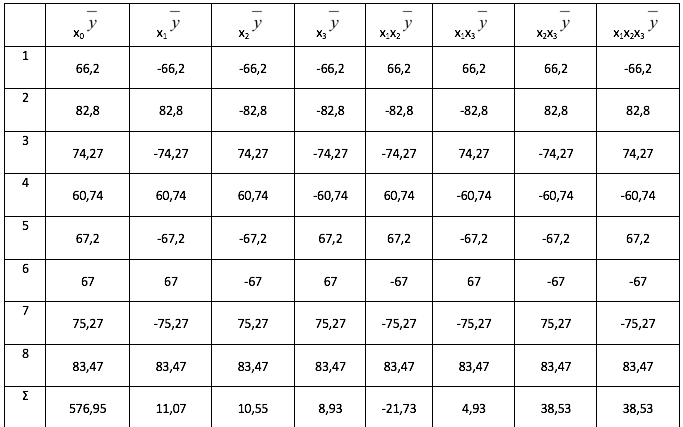
The coefficients of regression equations, formula (17) - (24) are found according to the formula (5)
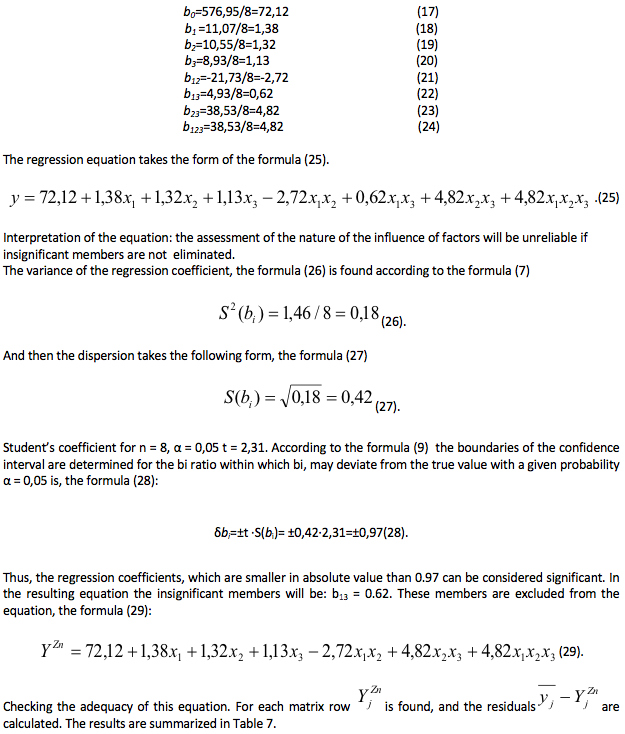
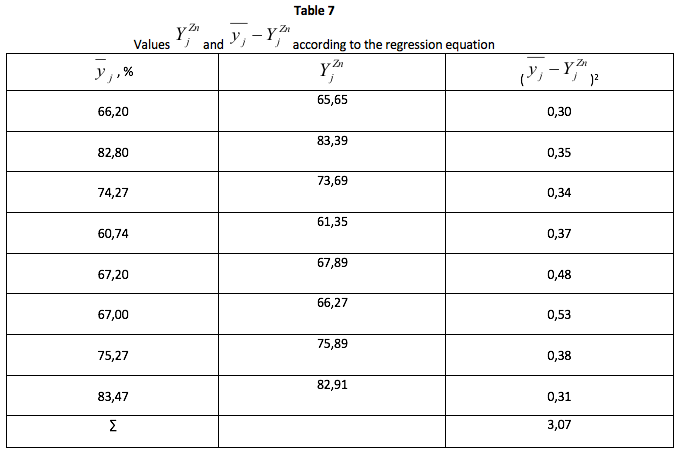
The residual variance, the formula (30) is defined according to the formula (10).
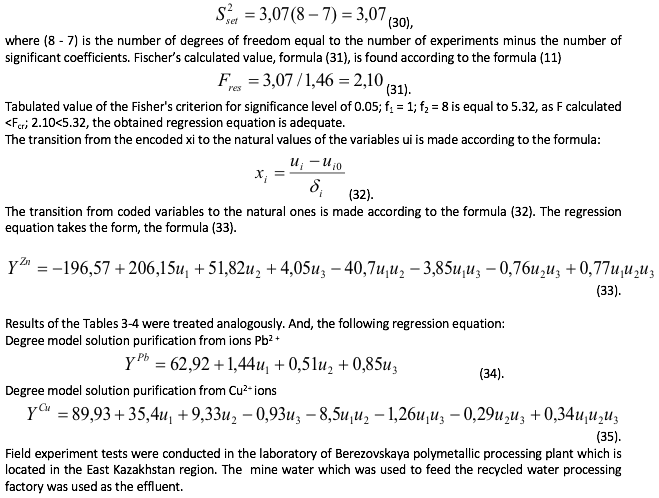

Table 8
Activation of the bentonite by 20% sulfuric acid and by neutralization
of aqueous bentonite slurry of Horizon 11, the experiment series No.1
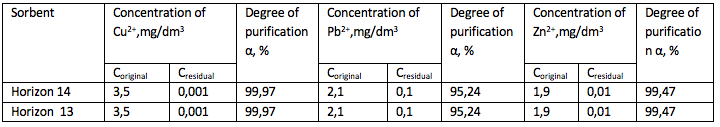
------
Table 9
Activation of the bentonite by 20% sulfuric acid and by neutralization
aqueous bentonite slurry of Horizon 11, the experiment series No.2
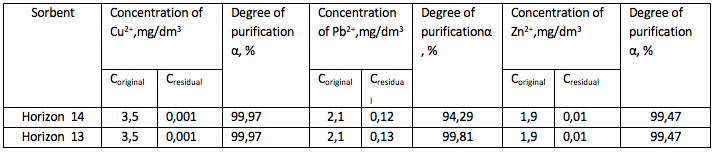
-----
Table 10
Activation of the bentonite by 20% sulfuric acid and by neutralization by
neutralization aqueous bentonite slurry of Horizon 11, the experiment series No.3
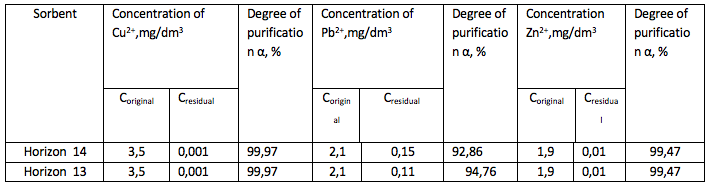
The uses of acid-alkaline which were activated by the bentonite in the purification of industrial wastewater from heavy metals will improve cleaning efficiency, and the resulting equations will allow to calculate the required amount of sorbent, and thereby to reduce the consumption of sorbents.
The technology of production of silica-alumina, bentonite sorbents in the form of a sorption-active paste of bentonite subjected to acid-alkaline activation was developed.
A mathematical model of sorption purification of effluent from the heavy metal ions (Cu2 +, Pb2 +, Zn2 +) was built.
The degree of purification depends on the initial concentration of the solution, the mass of sorbent and time of contact of sorbent with the solution.
The regression equations describing the process of cleaning wastes in the effluents from heavy metals in the f (Cu2 +, Pb2 +, Zn2 +) were derived.
Pilot-scale studies on the cleaning in the Berezovskaya Polymetaliic concentrator.
The results showed that the degree of purification from Pb2 + ions – 92.86%, Zn2+ - 99.47% and Cu 2+ 99.97%.
Battalova Sh. B. (1980). Catalysts and adsorbents based on bentonite Taganskii deposit and possible areas of application. Bentonite. Nauka. №2, 97p.
Mamchenko, A.V., Gerasimenko, N.G., Deshko, I.I., Pustovit, V.M., Pahar', T.A. (2008). Mixed chlorinated coagulant - reagent for natural and waste waters. Chemistry and technology of water.30 (3): 328 – 338.
Sautin S. N. (1975). Design of the Experiment in chemistry and chemical engineering [Text] / SN Sautin. - L .: Chemistry. – 48 p.
Uzdenbaeva ZH.K. (2014). Development of technology for treatment of industrial waste by sorbents. - Germany: LAP Lambert Academic Publishing. - 173 p.
Uzdenbaeva Zh.K. (2015). Treatment of industrial wastes bentonites // International journal of academic research, Vol. 7. No. 2. March, pp 105-108.
Uzdenbaeva Zh.K. (2014). Reducing Pollution of the Transboundary Irtysh River by Means of Industrial wastewater treatment by natural sorbents. 4: 137-144.
Uzdenbaeva, Zh.K., Adryshev, A.K., Hajrullina, A.A., Kravchenko, M.M. (2012). Sorbent based on aluminum compounds: an innovative patent for an invention. Republic of Kazakhstan, B01J20/16. Pat. 25542 Bulletin № 3. Publ. 15.03.2012.
1. Mathematical studies in ecology. Department of vocational training and technical subjects. Zhetysu state University named after I. Zhansugurov. Natural-technical faculty. Taldykorgan.king_bara@mail.ru
2. Academician of the International Academy of Informatization (2011), corresponding member of the Russian Academy of natural Sciences (2013). Zhetysu state University named after I. Zhansugurov. The physics and mathematics faculty. Taldykorgan.king_bara@mail.ru
3. Mathematical studies in ecology. Head of distance learning center. Academy of economics and Law named U. Zholdasbekov. Taldykorgan.king_bara@mail.ru